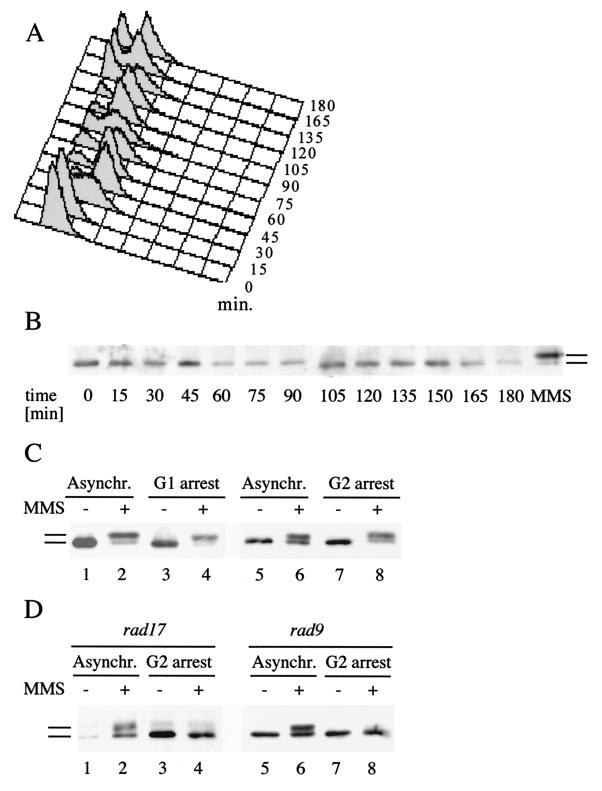
FIG. 3.
Rad55p phosphorylation in response to DNA damage during the cell cycle. (A) FACS analysis of a synchronized cell culture after α-factor arrest and release. At the indicated time intervals, aliquots were withdrawn, stained with propidium iodide, and analyzed by FACS. (B) Rad55p is not detectably phosphorylated during a normal cell cycle. At the same time intervals as in panel A, the Rad55p phosphorylation status was determined as described in the legend to Fig. 1. The rightmost lane (labeled MMS) shows a positive control (2 h with 0.1% MMS), indicating the migration behavior of phosphorylated Rad55p. (C) Rad55p phosphorylation in G1- and G2-arrested cells. Wild-type cells (FF181268) arrested either in G1 by α-factor (lanes 3 and 4) or in G2 by nocodazole (lanes 7 and 8) were treated with 0.1% MMS for 2 h (lanes 4 and 8) or left untreated (lanes 3 and 7). The Rad55p status was analyzed as described in the legend to Fig. 1. As a control, cycling wild-type cells were analyzed before (lanes 1 and 5) and after (lanes 2 and 6) MMS exposure. Asynchr., asynchronous. (D) Rad55p phosphorylation in G2-arrested cells is fully dependent on RAD9 or RAD17. The Rad55p status was analyzed as in panel A in rad9Δ cells (FF181270) left asynchronous (Asynchr.) (lanes 1 and 2) or arrested in G2 by nocodazole (lanes 3 and 4) and in rad17Δ cells (WDHY1236) left asynchronous (lanes 5 and 6) or arrested in G2 by nocodazole (lanes 7 and 8) after treatment with 0.1% MMS (lanes 2, 4, 6, and 8) or without MMS (lanes 1, 3, 5, and 7). Bars refer to the different forms of Rad55p.