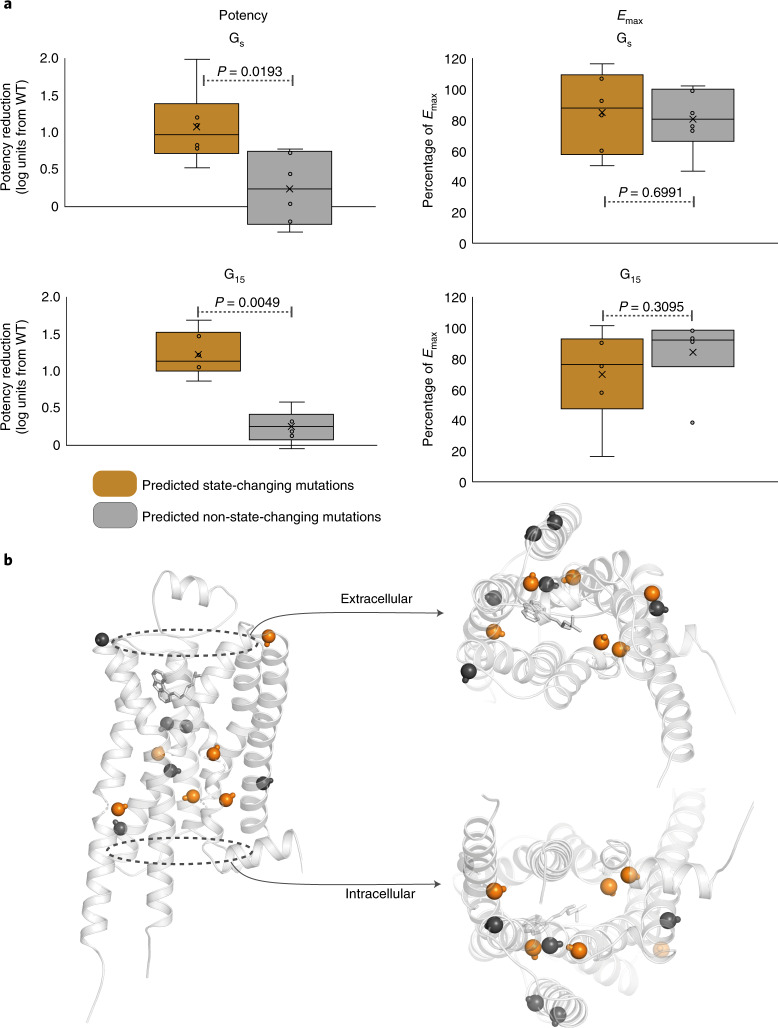
Fig. 5. Mutations of predicted state-changing residue positions reduce potency.
a, Effect upon alanine mutation of predicted state-changing and nonstate-changing residues, respectively, on epinephrine-induced β2-adrenoceptor activation of Gs and G15 measured by BRET-based biosensors. Predicted state-changing residues show a significantly higher reduction in potency (left), but not in efficacy (E, right), for both Gs and G15 relative to wild type. Statistical significance has been assessed by a two-sided Wilcoxon rank-sum test (n = 6 for each category, individual data points in Supplementary Table 2). Box-and-whiskers plots are presented with interquartile box bounds (25% and 75%); middle line represents the median; x represents the mean; whiskers extend to the minimum and maximum value. b, Structural mapping of predicted state-changing (orange) and nonstate-changing mutations (gray) on the inactive carazolol-bound inactive β2-adrenoceptor structure (PDB 2RH1)44. Cα are shown as spheres and Cα-Cβ bonds are displayed as sticks. Five out of six state-changing mutations cluster tightly in the transduction pathway between the ligand and G-protein pockets.