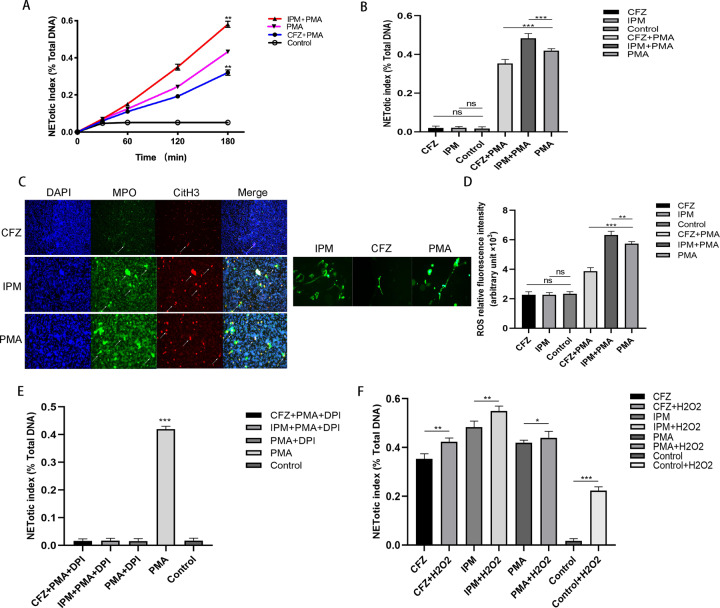
Fig. 6. The effects of antibiotics on ROS generation and NETs formation.
Human peripheral neutrophils were preincubated with antibiotics or PBS for 2 h and stimulated to form NETs with or without PMA (100 nM) for 3 h. A Kinetics of NETs releasing in PMA-stimulated neutrophils was recorded (n = 3). B NETs release in activated (with PMA) or resting (antibiotics alone) neutrophils was measured using Sytox Green fluorescence plate reader assay at 3 h and expression as the ratio of the percentage of total DNA (n = 3). Both antibiotics group showed effects on the NETs formation only in activated neutrophils. C To further confirm the existence of NETs, neutrophils were preincubated with antibiotics or PBS for 2 h and stimulated to form NETs with PMA (100 nM) for 3 h. DNA (blue) stained with DAPI, MPO (green) stained with anti-MPO antibody, and citH3 (red) stained with anti-citH3 were counterstained in neutrophils for immunofluorescence. DNA stained with Sytox Green alone in neutrophils for immunofluorescence to show the morphology of NETs. D Neutrophils were preincubated with β-Lactams (2 mM) for 2 h and then treated with or without PMA. Intracellular ROS were measured with DHR123 at 45 min (n = 3). E Neutrophils pre-cultivated with DPI, an NADPH oxidase inhibitor, were incubated with β-Lactams for 2 h and then activated with PMA. The ROS production was measured using DHR123 fluorescence plate reader assay at a time of 45 min (n = 3). F To confirm the role of ROS, exogenous H2 O2 (30 μM) was added after 2-h incubation with β-Lactams. NETosis was measured using Sytox Green fluorescence plate reader assay at the time of 3 h and expression as relative fluorescence unit (n = 3). Images are representative of three independent experiments. Bars were shown in the figure; data were analyzed by Student’s t-test. *P < 0.05, ** P < 0.01, ***P < 0.001, ns no significance. DE de-escalation. ES escalation.