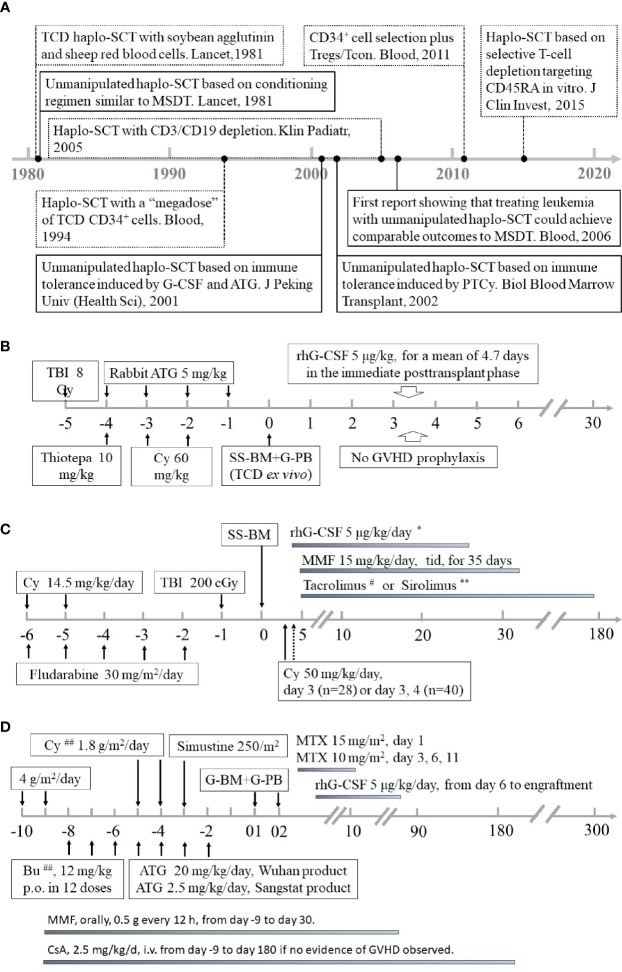
Figure 1.
Summary of historical perspective and conditioning regimens of different haploidentical stem cell transplantation modalities for AML. (A) The historical perspective of haplo-SCT for AML. (B) University of Perugia: myeloablative conditioning and ex vivo TCD with “megadose” CD34+ cell allografts. (C) Peking University: myeloablative conditioning based on immune tolerance induced by G-CSF and ATG. (D) Johns Hopkins University: nonmyeloablative conditioning with high-dose PT/Cy. Panels (B–D) were adapted from Aversa et al. (Blood, 1994), Luznik et al. and Cieri et al. (Biol Blood Marrow Transplant,2008; Biol Blood Marrow Transplant,2015), and Huang et al. (Bone Marrow Transplant,2006), respectively. AML, acute myeloid leukemia; Haplo-SCT, haploidentical stem cell transplantation; Tregs, regulatory T cells; Tcon, conventional T cells; MSDT, human leukocyte antigen (HLA)-matched sibling donor transplantation; G-CSF, granulocyte colony-stimulating factor; ATG, anti-thymocyte globulin; PTCy, posttransplantation cyclophosphamide; TBI, Total body irradiation; SS-BM, steady-state bone marrow; G-PB, G-CSF mobilized peripheral stem cells; GVHD, graft-versus-host disease; MMF, mycophenolate mofetil; G-BM, G-CSF-stimulated bone marrow harvests; MTX, Methotrexate; Bu, Busulfan; CSA, Cyclosporin A *Subcutaneous injection starting on Day 4 and continuing until recovery of neutrophils to >1000/μl for 3 days. #Tacrolimus was initiated at a dose of 1 mg i.v. daily, adjusted to achieve a therapeutic level of 5–15 ng/mL, and then converted to oral form until discontinuation. If there was no active GVHD, tacrolimus was tapered off by Day 180. **Sirolimus (orally, monitored 2 times each week to maintain a target therapeutic plasma level of 8 to 14 ng/mL during the first 2 months after transplantation, thereafter of 5 to 8 ng/mL until discontinuation). ##Patients 50 years old or older were conditioned with the same regimen as in (D), except for lower dosages of Bu (6–8 mg/kg) and Cy (1.0 g/m2/d).