Abstract
Rice cake is a traditional food in Korea, and is made by rice alone, or with other grain powder. To improve the health benefits of fermented rice cake, the rice powder was supplemented with strawberry powder. Anti-inflammatory activities of the mixture of strawberry and rice powder were evaluated. Treatment with the mixture significantly decreased the production of nitric oxide (NO). The mixture of strawberry and rice powder in the ratio 10: 90 effectively and dose-dependently reduced the immune-associated genes iNOS, IL-1β, IL-6, COX-2, and TNF-α. Furthermore, carrageenan-injected mice were used to study the anti-inflammatory effect of the mixture. Pre-oral administration of the mixture of strawberry and rice powder at doses of 50 and 100 mg/kg BW significantly reduced paw edema induced by carrageenan. These results suggest that for fermented rice cake production and processing, the strawberry and rice powder mixture may be a potential source of anti-inflammatory activity.
Keywords: Strawberry powder, Rice powder, Anti-inflammation, Carrageenan-induced paw edema, Rice cake
Introduction
The inflammatory process arises from microbial infection or tissue damage in the physiological system (Cheung et al., 2016), and long-term inflammation becomes an annoyance (Ferrero-Miliani et al., 2007). This inflammation is associated with various diseases and diverse chronical conditions, like aging (Panickar and Jewell, 2015), allergic reaction, stroke (Skaper et al., 2014), obesity (Donath et al., 2013), and depression (Ross, 1999). However, the body’s immune system defends against those infections, in which the production of nitric oxide (NO) and prostaglandins derived from arachidonic acid are key mediators to regulate the inflammatory process in which iNOS is expressed (Seibert et al., 1995; Wong and Billiar, 1995). Additionally, IL-1β, IL-6, and TNF-α are major genes in inflammatory modulation (Dinarello, 2000). This anti-inflammatory action to balance those inflammations in our immune system must always be maintained to protect our body systems from many critical diseases, such as inflammatory bowel disease, cancer, and diabetes (Crusz and Balkwill, 2015; Okamoto and Watanabe, 2016; Pickup, 2004).
Strawberry (Fragaria ananassa) was found to have many physiological activities. Polysaccharide extracted from strawberry showed anti-inflammatory and anti-apoptotic effects on lipopolysaccharide (LPS)-stimulated RAW264.7 through decreasing pro-inflammatory cytokines. In addition, strawberry polysaccharide modulated Bak and Bcl-2 protein levels in RAW264.7 cells (Liu and Lin, 2012). Polyphenolics extracted from strawberry also exhibited anti-inflammatory activities, and suppressed the expression of IL-1β, IL-6, and iNOS genes (Van de Velde et al., 2019). It was reported that strawberry contains an immuno-modulatory activity via stimulating the female BALB/c mice splenocyte proliferation (Lin and Tang, 2007). Another study showed the anti-inflammatory effect of strawberry juice on LPS-stimulated murine macrophage cells, in which strawberry juice inhibited LPS-induced inflammation via reducing pro-inflammatory cytokines and increasing anti-inflammatory cytokine secretions (Lin and Tang, 2008).
Rice is one of the globally important staples as a major energy and nutrient source, especially in Korea (Kaur et al., 2016), and rice cake is a famous traditional food in Korea. Rice cake is made from rice alone, or with other grain powder (Kim et al., 2014). However, the consumption of rice products have recently decreased in Korea, while many chronic diseases, such as obesity and diabetes, have increased, because of the change of dietary habits associated with Westernization (Kim et al., 2014; Pingali, 2007); thus the development of new rice products with health benefits is necessary. Rice cakes carry nutritional value as a single meal served as a highly convenient food product (Ku et al., 2018). It is reported in clinical study that the consumption of brown rice cakes over a one-week period reduced incremental insulin concentrations, plasma ghrelin, and a circulating appetite-promoting hormone (Pottgen et al., 2015). Therefore, the current study investigated the potential to improve the quality of fermented rice cake materials by including a mixture of rice and strawberry powder with anti-inflammatory activities, according to the addition of strawberry powder.
Materials and methods
Optimization of the concentrations of strawberry and rice powder
The strawberry and rice powders in all experiments were provided by Sangwhaepeuaenbi Co. Ltd. (Gangneung, Korea). RAW264.7 macrophage cells in RPMI-1640 medium (Gibco™, Waltham, MA, USA), supplemented with 10% fetal bovine serum (Welgene, Daegu, Korea) and 1% antibiotics (penicillin and streptomycin) (Welgene), were seeded in 96-well plates at the concentration of 1 × 105 cells/well. The plate was incubated at 37 °C in a humidified atmosphere of 5% CO2 for 24 h. The culture medium was discarded, and cells were pre-treated with different concentrations of strawberry or rice powder of 0, 0.39, 0.78, 1.56, 3.12, 6.25, and 12.50 mg/mL. After 1 h, cells were stimulated with or without 1 μg/mL of LPS (from Escherichia coli, Sigma–Aldrich, St. Louis, MO, USA), and plate was incubated for an additional 24 h. NO production in the cultured medium was measured using Griess reagent (Sigma–Aldrich). The stimulated cells were used to investigate cell proliferation using the EZ-Cytox Cell Viability Assay kit (Daeil Labservice, Seoul, Korea). NO production and cell proliferation were observed by microplate reader (EL800; BioTek, Winooski, VT, USA) at the absorbance of A540 and A450 nm, respectively. The same experiment was performed independently three times.
Optimization of the mixture ratio of strawberry and rice powder
Rice powder was mixed with strawberry powder in the ratios of strawberry powder: rice powder (10: 90, 7.5: 92.5, 5: 95, and 2.5: 97.5). The anti-inflammatory effects of the mixture of strawberry and rice powder were evaluated by the production of NO and the proliferation of cells. The experiment was performed as described above. Briefly, cells were pre-treated with various concentrations (0.78, 1.56, 3.12, and 6.25 mg/mL) of each ratio of the mixture for 1 h. Cells were stimulated with or without 1 μg/mL of LPS. After 24 h stimulation, the supernatant was used for NO production analysis, while the cultured cells were used for cell proliferation assay. The experiment was conducted in three independent trials.
Total RNA extraction and first stand cDNA synthesis
Suitable ratio of strawberry and rice powder was selected from previous observation (optimization of the mixture ratio of strawberry and rice powder). Recent studies have indicated that L-glutamine inhibited the inflammatory processes (Funakoshi et al., 2012; Jeong et al., 2018). Therefore, the mixture of L-glutamine (Sigma-Aldrich) and rice powder was used as the positive control in this experiment. Total RNA was extracted from the cells using Tri reagent® (Molecular Research Center, Cincinnati, OH, USA). The extracted RNA concentration was measured by nanophotometer (Implen, Munich, Germany). RNA (500 ng) was used to prepare the first stand cDNA using the High-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA, USA), according to the manufacturer’s instructions.
Immune-associated genes expression of strawberry and rice powder mixture
The expression of immune-associated genes was analyzed using SYBR® Premix Ex Taq™ II (Takara Bio Inc., Shiga, Japan) in a QuantStudio™ 3 Flex Real-Time PCR System (ThermoFisher Scientific, Waltham, MA, USA). The Real-Time PCR reaction mixture consisted of 10 ng of cDNA and 0.4 μM of each specific primer set (Table 1). The expression of immune-associated genes was quantified using QuantStudio™ 3 Flex Real-Time PCR software.
Table 1.
Sequences of oligonucleotide primers used for macrophage immune-associated genes test
| Gene | Accession No | Sequence |
|---|---|---|
| iNOS | BC062378.1 | Forward primer: TTCCAGAATCCCTGGACAAG |
| Reverse primer: TGGTCAAACTCTTGGGGTTC | ||
| IL-1β | NM_008361.4 | Forward primer: GGGCCTCAAAGGAAAGAATC |
| Reverse primer: TACCAGTTGGGGAACTCTGC | ||
| IL-6 | NM_031168.2 | Forward primer: AGTTGCCTTCTTGGGACTGA |
| Reverse primer: CAGAATTGCCATTGCACAAC | ||
| COX-2 | NM_011198.4 | Forward primer: AGAAGGAAATGGCTGCAGAA |
| Reverse primer: GCTCGGCTTCCAGTATTGAG | ||
| TNF-α | D84199.2 | Forward primer: ATGAGCACAGAAAGCATGATC |
| Reverse primer: TACAGGCTTGTCACTCGAATT | ||
| β-actin | NM_007393.5 | Forward primer: CCACAGCTGAGAGGGAAATC |
| Reverse primer: AAGGAAGGCTGGAAAAGAGC |
Anti-inflammatory effect of strawberry and rice powder mixture on carrageenan-induced paw edema mice
The anti-inflammatory effect of strawberry and rice powder was studied using mouse model, following the method of Yeşilada and Küpeli (2002). Male ICR mice with 28 ± 2 g of body weight were purchased from Orient Bio Inc., South Korea. Mice were maintained in a specific pathogen-free at temperature of RH at 20 ± 2 and 60 ± 5%, respectively. These experimental protocols were approved by the Gangneung-Wonju National University Committee. After a 1 week environmental adaptation period, mice were randomly separated into 6 groups (n = 5). Strawberry and rice mixture (50 and 100 mg/kg body weight (BW), strawberry powder (10 mg/kg BW), rice powder (90 mg/kg BW), and L-glutamine (10 mg/kg BW) were administrated orally. After 1 h administration, the freshly prepared suspension of 0.5 mg/25 μL carrageenan (Sigma–Aldrich, Missouri, USA) was injected into subplantar tissue of the right hind paw of each mouse. The left hind paw was used as the control, which was injected with saline solution. The size of paw edema was measured every 90 min for 6 h after carrageenan-induced paw using Digimatic Caliper (Mitutoyo Corporation, Kanagawa, Japan). The difference between right and left paw thickness was calculated and recorded.
Statistical analysis
Statistical analysis was performed with ‘Statistix 8.1’ Statistics Software. The results were compared using One-way ANOVA (Holm–Sidak post hoc multiple comparison test), and differences were considered significant at p < 0.05.
Results and discussion
Effect of the strawberry and rice powder on macrophage proliferation and NO production
Macrophages are one of the important immune cells that play a central role in a host’s defense against bacterial infection (Yun et al., 2008), and are involved in acute and chronic inflammatory responses (Olefsky and Glass, 2010). Macrophages also produce NO, and are critical in the antibacterial and antitumor defense system of innate immune cells (Underhill et al., 1999). In macrophages, LPS is known to induce inflammatory responses via increasing pro-inflammatory mediators and cytokines (Cho et al., 2016). Strawberry extract was demonstrated to inhibit NO production in LPS-stimulated RAW264.7 cells (Gasparrini et al., 2017).
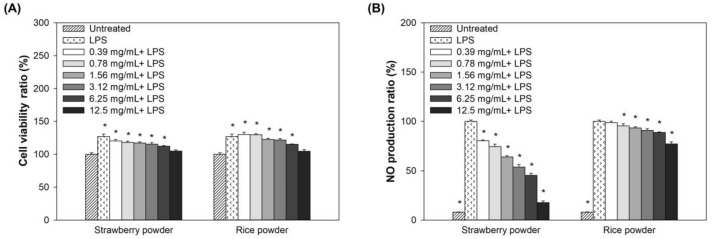
To investigate the anti-inflammatory effect of strawberry and rice powder, RAW264.7 macrophage cells were pre-treated with several concentrations of strawberry or rice powder, followed by LPS stimulation. Our results showed that up to 12.5 mg/mL, neither the strawberry nor the rice powder gave any cytotoxicity to the cells (Fig. 1A). Moreover, the strawberry and rice powder exhibited anti-inflammatory effects via significantly reduced production of NO in a dose-dependent manner (Fig. 1B). This finding is similar to the report by Rutledge et al. (2019) that the freeze-dried whole strawberry powder participants showed reduced NO production. Strawberry and rice powder gave the anti-inflammatory effect by reducing the LPS-induced NO production, so that in this paper, the synergistic effects of strawberry and rice powder combination experiments were performed.
Fig. 1.
Effect of strawberry and rice powder on LPS-stimulated RAW264.7 macrophage cell. (A) Cell proliferation, and (B) NO production. Significant differences are p < 0.05, compared with untreated group or LPS group (*)
Synergistic effect of strawberry and rice powder combination on macrophage proliferation and NO production
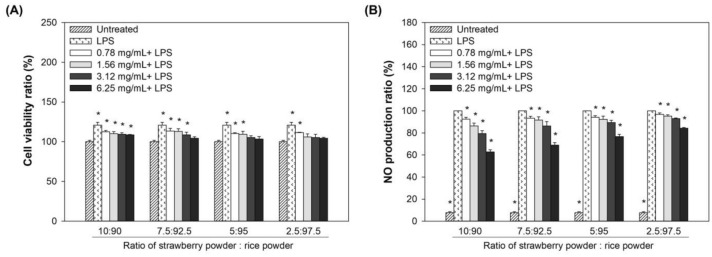
To evaluate the synergistic anti-inflammatory effect of the mixture of strawberry and rice powder combination, strawberry powder was mixed with rice powder to make the different ratios (10: 90, 7.5: 92.5, 5: 95, and 2.5: 97.5) of strawberry powder and rice powder. Based on the results of the treatment of the mixture of strawberry and rice powder on the level of LPS-induced NO, the synergistic effects of both components were evaluated at the concentrations (0.78, 1.56, 3.12, and 6.25 mg/mL) of each mixture. Figure 2A shows that up to 6.25 mg/mL, the mixtures of strawberry and rice powder did not give any cytotoxicity on RAW264.7 macrophage cells. The NO production was significantly reduced according to the concentration (Fig. 2B) of the mixtures, and the pre-treatment of the mixture at the ratio of 10: 90 gave the best production of NO, and that ratio was selected for the next experiment.
Fig. 2.
Effect of various ratios of strawberry and rice powder mixture on LPS-stimulated RAW264.7 macrophage cell. (A) Cell proliferation, and (B) NO production. Significant differences are p < 0.05, compared with untreated group or LPS group (*)
Effect of strawberry and rice powder mixture on the immune-associated gene expression level
The immune systems in macrophages are regulated via the expression of pro-inflammatory cytokines and associated genes (IL-1β, IL-6, and TNF-α) (Gopinath et al., 2006; Yencilek et al., 2015). LPS is known to stimulate the release of pro-inflammatory cytokines in RAW264.7 cells (Waseem et al., 2008). Pre-treatment of the mixture with the ratio 10: 90 (strawberry powder and rice powder) at the concentration ranging 0.78 to 6.25 mg/mL for 1 h on RAW264.7 cells before LPS stimulation resulted in a significant suppression of IL-1β, IL-6, COX-2, and TNF-α (Fig. 3B–E) expression. LPS is also known to induce the expression of iNOS, and causes the release of NO in the macrophage cells (Kim et al., 2002). Strawberry and rice powder mixture remarkably decreased LPS-induced NO production (Fig. 2B), as well as remarkably inhibiting iNOS gene production (Fig. 3A). In addition, pro-inflammatory cytokine expression levels in the strawberry and rice powder mixture-treated groups were similar to or lower than that observed in the mixture of L-glutamine and rice powder as a positive control group. This suggests that the mixture of strawberry and rice powder modulates immune responses via suppressing the expression of pro-inflammatory cytokines.
Fig. 3.
Effect of strawberry and rice powder mixture on quantification of immune associated genes in relative expression (fold), compared with untreated group. (A) Relative expression of iNOS, (B) Relative expression of IL-1β, (C) Relative expression of IL-6, (D) Relative expression of COX-2, and (E) Relative expression of TNF-α. Significant differences are p < 0.05, compared with LPS group (*)
Effect of the strawberry and rice powder mixture on carrageenan-induced paw edema
Mouse paw edema has been widely used to evaluate the effect of new anti-inflammatory drugs (Posadas et al., 2004), and carrageenan is a family of polysaccharides that have been used to induce inflammation (Myers et al., 2019). The carrageenan administration in rats caused increased levels of PGE2, IL-1β, IL-6, and TNF-α (Vazquez et al., 2015), and localized edema (Fulgenzi et al., 2005). Moreover, the carrageenan-induced paw edema in mouse also raised the production of PEG2 (Lee et al., 2010) and various pro-inflammatory cytokines (Ou et al., 2019) in the serum, COX-1, COX-2, and iNOS proteins (Posadas et al., 2004). The current study evaluated the anti-inflammatory effect of the mixture of strawberry and rice powder on carrageenan-induced paw edema under in vivo ICR mice system. Mice received the treatments, and paw edema in mice was induced by carrageenan injection. Table 2 shows that the injection of carrageenan led to paw edema in ICR mice over a period of 6 h. The treatment of L-glutamine, strawberry powder, and rice powder has also been observed to have anti-inflammatory effect after 270 min post carrageenan injection (Table 2). However, administration of the mixture of strawberry and rice powder with 50 and 100 mg/kg BW differently decreased the size of right and left paw edema after 180 min post-carrageenan induction (Table 2). The anti-inflammatory activity of the strawberry and rice powder mixture gave similar reducing time (after 180 min) as the activity of Berberidacceae roots extracts (Ivanovska and Philipov, 1996). Moreover, the anti-inflammatory activity on paw edema of strawberry and rice powder mixture can be observed to be faster than the effect of Berberis root and bark extract (Yeşilada and Küpeli, 2002).
Table 2.
The effect of mixture strawberry and rice powder on different size between left and right paw (mm) of carrageenan-induced paw edema
| Treatment | Dose (mg/kg BW) |
Time after carrageenan-induced (min) | |||
|---|---|---|---|---|---|
| 90 | 180 | 270 | 360 | ||
| Untreated | – | 0.63 ± 0.032 | 0.94 ± 0.031 | 1.24 ± 0.067 | 1.35 ± 0.035 |
| L-glutamine | 10 | 0.46 ± 0.056* | 0.75 ± 0.070* | 1.03 ± 0.078* | 0.82 ± 0.038* |
| Strawberry powder | 10 | 0.75 ± 0.035* | 0.84 ± 0.046 | 1.04 ± 0.042* | 0.93 ± 0.031* |
| Rice powder | 90 | 0.64 ± 0.038 | 0.92 ± 0.051 | 1.10 ± 0.020* | 0.96 ± 0.053* |
| Strawberry powder: rice powder (10: 90) | 50 | 0.54 ± 0.050 | 0.87 ± 0.035 | 0.69 ± 0.071* | 0.62 ± 0.052* |
| 100 | 0.53 ± 0.075 | 0.76 ± 0.020* | 0.54 ± 0.045* | 0.40 ± 0.026* | |
*p < 0.05, when compared with saline group
The present study demonstrated that the mixture of strawberry and rice powder significantly decreased the LPS-induced NO production, and the pre-treatment of the mixture of strawberry and rice powder exhibited anti-inflammatory activity via significantly suppressing the expression of the immune-associated genes iNOS, IL-1β, IL-6, COX-2, and TNF-α. Moreover, the mixture of strawberry and rice powder in the concentration ratio 10: 90 also reduced the carrageenan-induced paw edema under in vivo ICR mice system. These results suggest that the mixture of strawberry and rice powder may be a potential source of anti-inflammatory activity for fermented rice cake production and processing.
Acknowledgements
This study was supported by the R&D Program (P0010716) of the Ministry of SMEs and Startups of Korea. This study is also supported by the University Emphasis Research Institute Support Program (No.2018R1A61A03023584), from the National Research Foundation of Korea.
Compliance with ethical standards
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chaiwat Monmai, Ju Hyun Nam, Jun Hyeok Lim authors equally contributed to the research.
References
- Cheung RCF, Ng TB, Wong JH, Chen Y, Chan WY. Marine natural products with anti-inflammatory activity. Applied Microbiology and Biotechnology. 2016;100:1645–1666. doi: 10.1007/s00253-015-7244-3. [DOI] [PubMed] [Google Scholar]
- Cho BO, Yin HH, Park SH, Byun EB, Ha HY, Jang SI. Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-kB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW264.7 macrophages. Bioscience, Biotechnology, and Biochemistry 80: 1520-1530 (2016) [DOI] [PubMed]
- Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nature Reviews Clinical Oncology. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Proinflammatory cytokines. American College of Chest Physicians. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- Donath MY, Dalmas É, Sauter NS, Böni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metabolism. 2013;17:860–872. doi: 10.1016/j.cmet.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clinical and Experimental Immunology. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulgenzi A, Dell'Antonio G, Foglieni C, Cin ED, Ticozzi P, Franzone JS, Ferrero ME. Inhibition of chemokine expression in rat inflamed paws by systemic use of the antihyperalgesic oxidized ATP. BMC Immunology. 2005;6:18–28. doi: 10.1186/1471-2172-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi T, Yamashita K, Ichikawa N, Fukai M, Suzuki T, Goto R, Oura T, Kobayashi N, Katsurada T, Ichihara S, Ozaki M, Umezawa K, Todo S. A novel NF-kB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic injury in mice. Journal of Crohn's and Colitis. 2012;6:215–225. doi: 10.1016/j.crohns.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Gasparrini M, Forbes-Hernandez TY, Giampieri F, Afrin S, Alvarez-Suarez JM, Mazzoni L, Mezzetti B, Quiles JL, Battino M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW264.7 macrophages. Food and Chemical Toxicology. 2017;102:1–10. doi: 10.1016/j.fct.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Gopinath VK, Musa M, Samsudin AR, Sosroseno W. Role of interleukin-1beta and tumour necrosis factor-alpha on hydroxyapatite-induced phagocytosis by murine macrophages (RAW264.7 cells) British Journal of Biomedical Science. 2006;63:176–178. doi: 10.1080/09674845.2006.11978094. [DOI] [PubMed] [Google Scholar]
- Ivanovska N, Philipov S. Study on the anti-inflammatory action of Berberis vulgaris root extract, alkaloid fractions and pure alkaloids. International Journal of Immunopharmacology. 1996;18:553–561. doi: 10.1016/S0192-0561(96)00047-1. [DOI] [PubMed] [Google Scholar]
- Jeong SY, Im YN, Youm JY, Lee HK, Im SY. L-Glutamine attenuates DSS-induced colitis via induction of MAPK phosphatase-1. Nutrients. 2018;10:288–297. doi: 10.3390/nu10030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur B, Ranawana V, Henry J. The glycemic index of rice and rice products: a review, and table of GI values. Critical Reviews in Food Science and Nutrition. 2016;56:215–236. doi: 10.1080/10408398.2012.717976. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kwon KJ, Park JY, Lee SH, Moon CH, Baik EJ. Effects of peroxisome proliferator-activated receptor agonists on LPS-induced neuronal death in mixed cortical neurons: associated with iNOS and COX-2. Brain Research. 2002;941:1–10. doi: 10.1016/S0006-8993(02)02480-0. [DOI] [PubMed] [Google Scholar]
- Kim DH, Lim YT, Park YJ, Yeon SJ, Jang KI. Antioxidant activities and physicochemical properties of tteokbokki rice cakes containing cinnamon powder. Food Science and Biotechnology. 2014;23:425–430. doi: 10.1007/s10068-014-0058-8. [DOI] [Google Scholar]
- Ku SK, Hong JS, Choi HD, Park JD, Kim YB, Choi HW, Kim TK, Choi YS. A study of the quality characteristics of frozen Korean rice cake by using different thawing methods. Food Science and Biotechnology. 2018;27:1343–1351. doi: 10.1007/s10068-018-0376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Chen LG, Liang WL, Wang CC. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chemistry. 2010;118:315–322. doi: 10.1016/j.foodchem.2009.04.123. [DOI] [Google Scholar]
- Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chemistry. 2007;101:140–147. doi: 10.1016/j.foodchem.2006.01.014. [DOI] [Google Scholar]
- Lin JY, Tang CY. Strawberry, loquat, mulberry, and bitter melon juices exhibit prophylactic effects on LPS-induced inflammation using murine peritoneal macrophages. Food Chemistry. 2008;107:1587–1596. doi: 10.1016/j.foodchem.2007.10.025. [DOI] [Google Scholar]
- Liu CJ, Lin JY. Anti-inflammatory and anti-apoptotic effects of strawberry and mulberry fruit polysaccharides on lipopolysaccharide-stimulated macrophages through modulating pro-/anti-inflammatory cytokines secretion and Bcl-2/Bak protein ratio. Food and Chemical Toxicology. 2012;50:3032–3039. doi: 10.1016/j.fct.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Myers MJ, Deaver CM, Lewandowski AJ. Molecular mechanism of action responsible for carrageenan-induced inflammatory response. Molecular Immunology. 2019;109:38–42. doi: 10.1016/j.molimm.2019.02.020. [DOI] [PubMed] [Google Scholar]
- Okamoto R, Watanabe M. Role of epithelial cells in the pathogenesis and treatment of inflammatory bowel disease. Journal of Gastroenterology. 2016;51:11–21. doi: 10.1007/s00535-015-1098-4. [DOI] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual Review of Physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Ou Z, Zhao J, Zhu L, Huang L, Ma Y, Ma C, Luo C, Zhu Z, Yuan Z, Wu J, Li R, Yi J. Anti-inflammatory effect and potential mechanism of betulinic acid on λ-carrageenan-induced paw edema in mice. Biomedicine & Pharmacotherapy. 2019;118:109347. doi: 10.1016/j.biopha.2019.109347. [DOI] [PubMed] [Google Scholar]
- Panickar KS, Jewell DE. The beneficial role of anti-inflammatory dietary ingredients in attenuating markers of chronic low-grade inflammation in aging. Hormone Molecular Biology and Clinical Investigation. 2015;23:59–70. doi: 10.1515/hmbci-2015-0017. [DOI] [PubMed] [Google Scholar]
- Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- Pingali P. Westernization of Asian diets and the transformation of food systems: Implications for research and policy. Food Policy. 2007;32:281–298. doi: 10.1016/j.foodpol.2006.08.001. [DOI] [Google Scholar]
- Posadas I, Bucci M, Roviezzo F, Rossi A, Parente L, Sautebin L, Cirino G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. British Journal of Pharmacology. 2004;142:331–338. doi: 10.1038/sj.bjp.0705650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- possible involvement of the IKK and MAPK pathways Yun KJ, Kim JY, Kim JB, Lee KW, Jeong SY, Park HJ, Jung HJ, Cho YW, Yun K, Lee KT. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-kappa B inactivation in RAW264.7 macrophages. International Immunopharmacology. 2008;8:431–441. doi: 10.1016/j.intimp.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Pottgen E, Yoon SH, Pham T, Gu X, Seo HS, Lee SO. Effects of Korean rice cake (Seolgitteok) on plasma glucose, insulin, and satiety hormones. The FASEB Journal 29: LB375 (2015)
- Ross R. Atherosclerosis-an inflammatory disease. New England Journal of Medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rutledge GA, Fisher DR, Miller MG, Kelly ME, Bielinski DF, Shukitt-Hale B. The effects of blueberry and strawberry serum metabolites on age-related oxidative and inflammatory signaling in vitro. Food & Function. 2019;10:7707–7713. doi: 10.1039/C9FO01913H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert K, Masferrer J, Zhang Y, Gregory S, Olson G, Hauser S, Leahy K, Perkins W, Isakson P. Mediation of inflammation by cyclooxygenase-2. Agents and Actions Supplements. 1995;46:41–50. doi: 10.1007/978-3-0348-7276-8_5. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Facci L, Giusti P. Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: a review. CNS & Neurological Disorders Drug Targets. 2014;13:1654–1666. doi: 10.2174/1871527313666141130224206. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- Van de Velde F, Esposito D, Grace MH, Pirovani ME, Lila MA. Anti-inflammatory and wound healing properties of polyphenolic extracts from strawberry and blackberry fruits. Food Research International. 2019;121:453–462. doi: 10.1016/j.foodres.2018.11.059. [DOI] [PubMed] [Google Scholar]
- Vazquez E, Navarro M, Salazar Y, Crespo G, Bruges G, Osorio C, Tortorici V, Vanegas H, López M. Systemic changes following carrageenan-induced paw inflammation in rats. Inflammation Research. 2015;64:333–342. doi: 10.1007/s00011-015-0814-0. [DOI] [PubMed] [Google Scholar]
- Waseem T, Duxbury M, Ito H, Ashley SW, Robinson MK. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery. 2008;143:334–342. doi: 10.1016/j.surg.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JM, Billiar TR. Regulation and function of inducible nitric oxide synthase during sepsis and acute inflammation. Advances in Pharmacology. 1995;34:155–170. doi: 10.1016/S1054-3589(08)61084-4. [DOI] [PubMed] [Google Scholar]
- Yencilek F, Yildirim A, Yilmaz SG, Altinkilic EM, Dalan AB, Bastug Y, Isbir T. Investigation of interleukin-1β polymorphisms in prostate cancer. Anticancer Research. 2015;35:6057–6061. [PubMed] [Google Scholar]
- Yeşilada E, Küpeli E. Berberis crataegina DC. root exhibits potent anti-inflammatory, analgesic and febrifuge effects in mice and rats. Journal of Ethnopharmacology. 2002;79:237–248. doi: 10.1016/S0378-8741(01)00387-7. [DOI] [PubMed] [Google Scholar]