FIGURE 7.
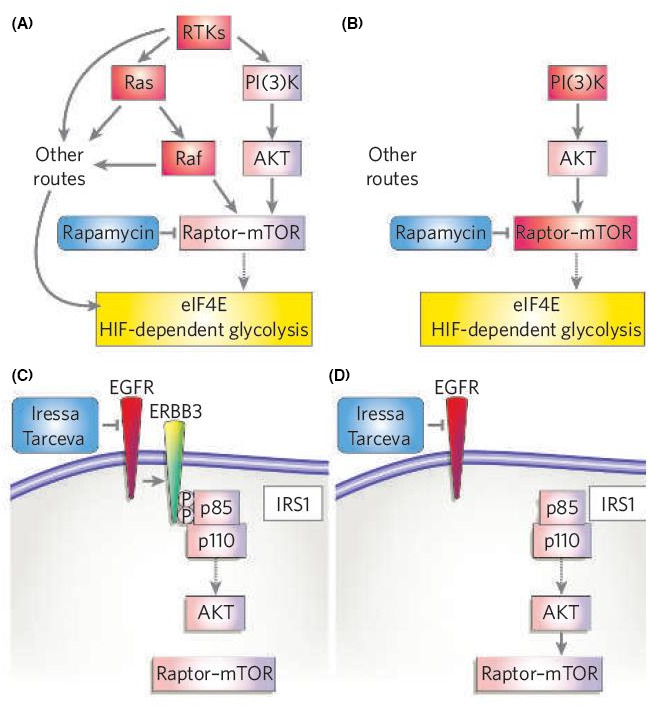
Pathway circuitry dictates therapeutic response. (A) For tumours with defined genetic lesions, the ability to overcome a given targeted therapeutic lies in whether or not they need to acquire a secondary genetic mutation to overcome the effect of the drug on critical downstream biochemical effectors that are required for continued tumour cell growth, or whether they can simply upregulate existing alternative routes that lead to effectors already expressed in those cells. So, the drug places selection pressure to ramp up existing bypass routes. If there are no such routes to the critical downstream effectors, a specific mutation to upregulate those alternative routes or bypass the drug are required. In this example, a critical target for tumour cell growth and survival is the activation of eIF4E and HIF. Tumours with initiating mutations in RTKs, Ras or Raf have multiple routes to signal to eIF4E and HIF, so blocking mTOR with rapamycin does not inhibit these tumours. (B) In contrast, tumours with initiating lesions in PI(3)K or more direct regulators of mTOR (such as LKB1 and TSC) do not have alternative routes to activate eIF4E and HIF. Hence these tumours show greater response to rapamycin. (C) Similarly, the expression and use of specific adaptor proteins that enhance certain arms of pathway signalling will dictate the therapeutic response. In the example shown, human lung tumours expressing epidermal‐growth‐factor receptor (EGFR) are targeted with anti‐EGFR drugs such as Iressa or Tarceva. In tumours expressing the ERBB3 heterodimerization partner, EGFR efficiently enhances PI(3)K activation through a number of PI(3)K‐binding sites in ERBB3. (D) In tumours that lack ERBB3, PI(3)K is still activated by a number of other routes, including adaptors such as insulin receptor substrate 1 (IRS1). Reprinted from [86]. Copyright© 2006, Nature
