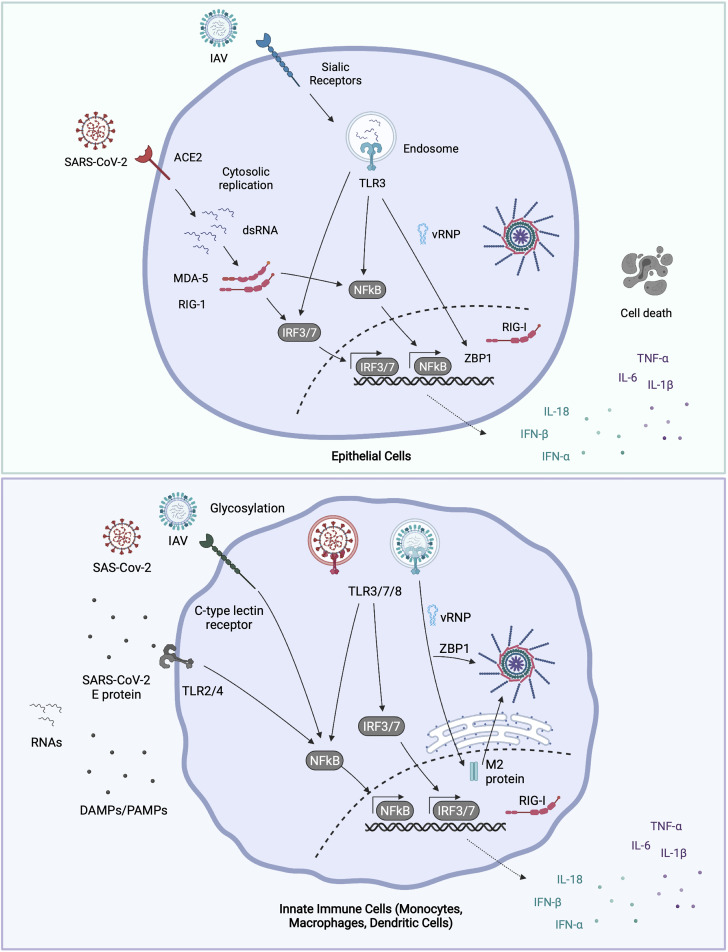
Figure 1.
Molecular sensing of IAV and SARS-CoV-2. IAV binds to surface α2, 6-linked sialic acid (α2,6-SA) receptors on airway epithelial cells, and some macrophages, whereupon it’s internalized into endosomes (76). Incoming virions may be sensed by TLR3 (epithelial cells) or by TLR3, 7 and 8 (macrophages and dendritic cells). TLR engagement leads to NFκB and IRF transcription factor activation and pro-inflammatory and anti-viral gene expression. Post fusion, viral RNA bound by nucleoprotein (vRNPs) are released into the cytosol. Once in the cytosol, vRNPs are trafficked to the nucleus where IAV replicates. IAV replication products are sensed by nuclear RIG-I or ZBP1, triggering gene expression or cell death respectively. ZBP1 may also intercept vRNAs in the cytosol and trigger inflammasome activation. Newly synthesized IAV M2 protein can trigger NLRP3 inflammasome activation and IL-1β release. SARS-CoV-2 enters airway epithelial cells via ACE2, and after fusion at or near the plasma membrane, the viral genome is released into the cytosol. The dsRNA intermediates generated during SARS-CoV-2 replication are sensed by both RIG-I and MDA-5 in the cytosol, leading to NFκB and IRF3 activation. Dendritic cells and macrophages do not appear to be productively infected with SARS-CoV-2 (71). Though whether they can still take up virus, and signal from endosomal TLRs isn’t known. TLR7 in plasmacytoid dendritic cells recognises either virions or ssRNAs from SARS-CoV-2. Extracellular viral PAMPS and DAMPS released from neighbouring infected cells can also be sensed by surface and endosomal TLRs [e.g., SARS-CoV-2 envelope ‘E’ protein: TLR2 (74)], driving NFkB activation. Glycosylated viral proteins can further be detected by CLRs expressed on macrophages, which drives NFκB activation (77). Figure created with BioRender.com.