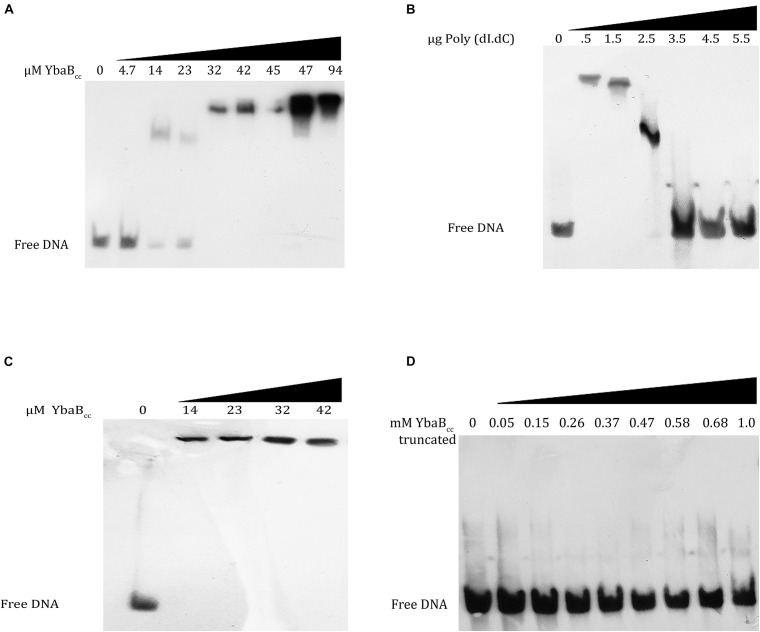
FIGURE 4.
YbaBCc binds to DNA in a sequence-independent manner in vitro. YbaBCc was incubated with 8 nM each of biotin-labeled probes. (A) b-WT (124 bp) from Borrelia burgdorferi strain B31 was incubated with increasing concentrations (0, 4.7, 14, 23, 32, 42, 45, 47, and 94 μM) of YbaBCc protein. (C) cc-WT (200 bp) Caulobacter probe was incubated with increasing concentrations (0, 14, 23, 32, and 42 μM) of YbaBCc protein. Reactions were electrophoresed on 6% DNA retardation gels and visualized by chemiluminescent detection. YbaBCc forms DNA–protein complexes with both probes (A–C) causing shifts of protein–DNA complexes. (B) Competitive binding was also studied by adding varied concentrations (0, 0.5, 1.5, 2.5, 3.5, 4.5, and 5.5 μg) of Poly (dI.dC) to YbaBCc (32 μM). The presence of free DNA with increasing Poly (dI.dC) indicates sequence independent DNA-binding activity of YbaBCc by exchange of b-WT (124 bp) from Borrelia burgdorferi strainB31 probe with Poly (dI.dC). (D) YbaBCc (Tn) protein (containing only N-terminal DNA-binding domain) was also tested for its ability to bind b-WT Borrelia probe. However, there was no shift observed even at very high protein concentrations rendering this truncated version of YbaBCc to be ineffective in DNA-binding suggesting that both DNA-binding domains are essential for YbaBCc protein to bind target DNA.