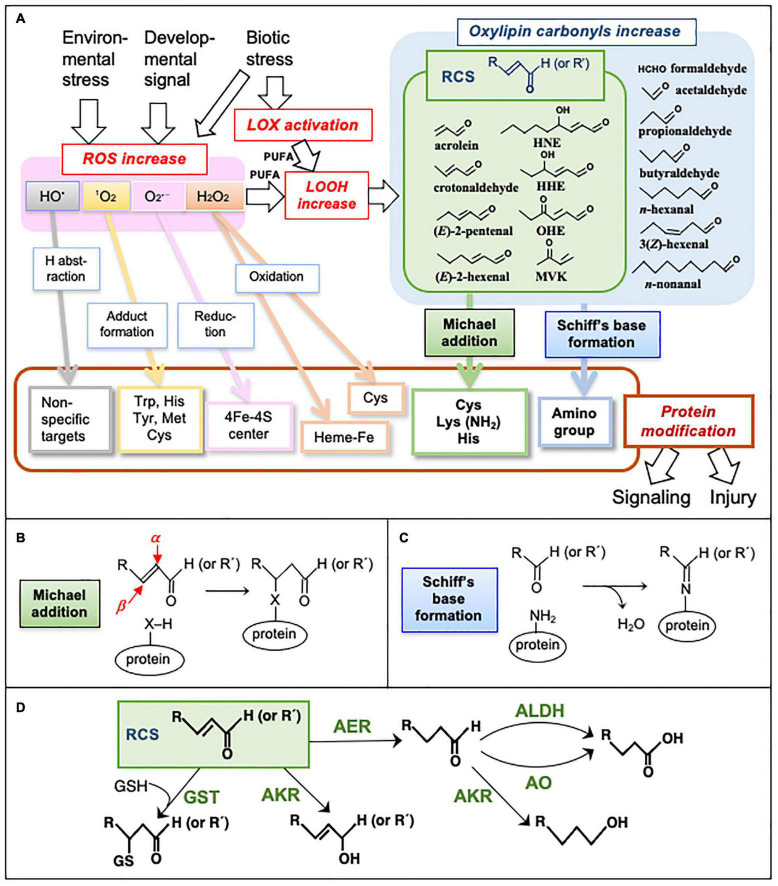
FIGURE 1.
Metabolism and reactions of oxylipin carbonyls. (A) Formation of ROS and oxylipin carbonyls and their actions causing signaling and injury via protein modification. HO• is highly reactive and non-specifically oxidizes almost all biomolecules. 1O2 is also highly reactive, and it prefers adduct formation on a double bond and a sulfur atom. O2•–, a relatively less reactive ROS, can reductively destroy the 4Fe-4S center in some enzymes such as aconitase (Halliwell and Gutteridge, 2015). H2O2 is also less reactive and may oxidize the Fe atom in the heme proteins such as ascorbate peroxidase or guaiacol peroxidase to inactivate them (Miyake and Asada, 1996). Oxylipin carbonyls and RCS, produced via the oxidation of PUFA by ROS, react with proteins in different manners from ROS. (B) Formation of the Michael adduct on a protein. The α- and β-carbons in an RCS molecule are indicated by red arrows. X, a nucleophilic atom. (C) Schiff’s base formation on a protein. (D) Enzymes to scavenge carbonyls and RCS in plants. AER, 2-alkenal reductase; AKR, aldo-keto reductase; ALDH, aldehyde dehydrogenase; AO, aldehyde oxidase; GST, glutathione transferase.