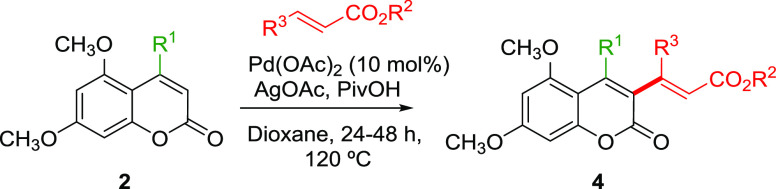
Table 5. Intermolecular C–H Alkenylation of Coumarins 2.
| entry | 2 | R1 | R2 | R3 | 4 | yield (%)a |
|---|---|---|---|---|---|---|
| 1 | 2a | Ph | CH3 | H | 4a | 31 |
| 2 | 2a | Ph | t-Bu | H | 4b | 31 |
| 3 | 2d | 4(tBu)C6H4 | CH3 | H | 4c | 52 |
| 4 | 2e | 3,4(OMe)C6H4 | CH3 | H | 4d | 28 |
| 5 | 2i | CH3 | CH3 | H | 4e | 45 |
| 6 | 2i | CH3 | t-Bu | H | 4f | 53 |
| 7 | 2i | CH3 | CH3 | CH3 | 4 g | 44 |
Yield (%) of the pure isolated compound; in all cases, 30–40% of the unreacted starting material was also recovered.