Abstract
Maternal vaccination is an effective means of protecting pregnant women, their fetuses, and infants from vaccine-preventable infections. Despite the availability of sufficient safety data to support the use of vaccines during pregnancy, maternal immunization remains an underutilized method of disease prevention, often because of concerns from both healthcare providers and pregnant women about vaccine safety. Such concerns have been reflected in the low uptake of the COVID-19 vaccine among pregnant women seen in many parts of the world. Here, we present an update of the current recommendations for the use of vaccines during pregnancy, including the evidence supporting the use of novel vaccine platforms. We also provide an overview of the data supporting the use of COVID-19 vaccines in pregnancy and an update of the status of vaccines that are currently under development for use in pregnant women.
Key words: COVID-19 vaccination, immunogenicity, maternal vaccination, neonates, safety
Introduction
Pregnancy and infancy are both periods of increased vulnerability to infection.1 Vaccinating women during pregnancy has been shown to be effective in providing protection against a number of infections in pregnant women, while also providing protection for the fetus and the infant during early life. Despite these benefits, low vaccine confidence remains a significant barrier to vaccine uptake among pregnant women worldwide and has been a particular challenge during the COVID-19 pandemic, which has seen low rates of vaccine uptake among this cohort. Although a small number of vaccines are recommended for routine use during pregnancy, there are many vaccines that have sufficient safety data to support their use in pregnant women in appropriate circumstances. In this review, we will provide an overview of the current recommendations and evidence supporting the use of vaccinations in pregnancy, including recommendations for the use of novel COVID-19 vaccines.
The Rationale for Vaccination During Pregnancy
Vaccinating women during pregnancy has 2 distinct potential benefits. Firstly, it protects the woman from infections that she may be particularly susceptible to during pregnancy, which in turn, protects the fetus from congenital infection and other harmful effects of maternal infection. Secondly, maternal vaccination may be used for the primary intention of protecting the developing fetus and infant from infection during the first months of life through the placental transfer of neutralizing immunoglobulin G (IgG) antibodies and/or secretory immunoglobulin A (IgA) antibodies in the mother’s breast milk (Figures 1 and 2 ).
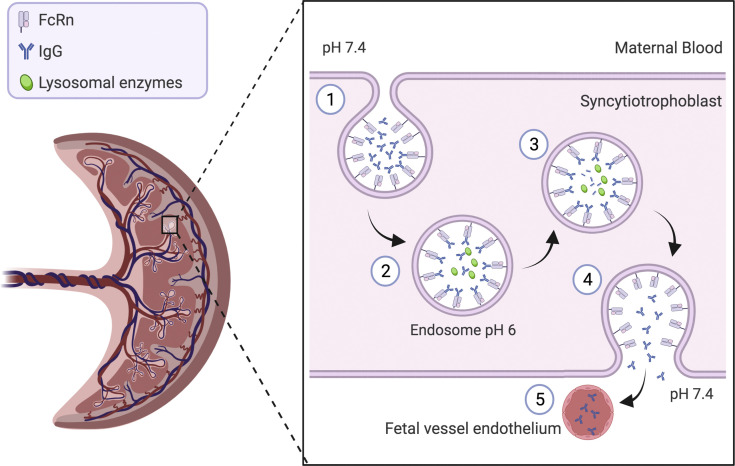
Figure 1.
Placental transfer of IgG antibodies from maternal to fetal circulation
Maternal IgG antibodies are taken up into endosomes within the syncytiotrophoblast cells of the placenta and bind to the FcRn. Following acidification of the endosome, the IgG antibodies are then transcytosed to the fetal side of the syncytiotrophoblast. The endosome fuses with the syncytiotrophoblast membrane, and the IgG antibodies are then released into the fetal circulation. The higher physiological pH within the fetal circulation promotes dissociation of the IgG from the FcRn (adapted from Palmeria et al).2 Figure created with BioRender.com, exported with publication and licensing rights. Original figure held under a Creative Commons license.
FcRn, neonatal Fc receptors; IgG, immunoglobulin G.
Etti. Maternal vaccination. Am J Obstet Gynecol 2022.
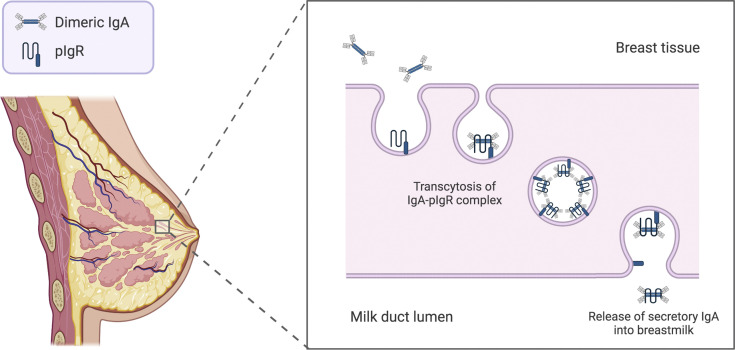
Figure 2.
Transfer of secretory IgA antibodies from maternal breast tissue to breast milk
Dimeric IgA molecules attach to the pIgR on the basolateral membrane of the mammary gland epithelium and are transcytosed through epithelial cells. At the apical cell membrane, the IgA dimer is released into the breast milk with a portion of the pIgR molecule (the secretory chain) still attached (adapted from Albrecht and Arck).3 Figure created with BioRender.com, exported with publication and licensing rights. Original figure held under a Creative Commons license.
IgA, immunoglobulin A; pIgR, polymeric Ig-receptors.
Etti. Maternal vaccination. Am J Obstet Gynecol 2022.
The benefit of maternal vaccination for infants was first demonstrated in 1879 when it was recognized that the infants born to women immunized against the vaccinia virus during pregnancy were immune to smallpox during early life.4 Neonatal vaccination is an alternative measure for the protection of infants from infection; however, it relies on the infant’s ability to produce neutralizing antibodies and is less likely to be effective in providing protection against pathogens during the first few weeks of life.5 Importantly, many vaccines are not administered to infants until they are at least 6 weeks of age and often require ≥2 doses before achieving full protection, thus leaving a critical gap where they are at an increased risk of infection. Vaccinating the mother during pregnancy can augment the transfer of maternal antibodies, thus narrowing the “window of vulnerability” to infections and prolonging the period of protection from disease.1
There are many currently licensed vaccines that provide protective immunity that is beneficial for both mothers and infants, such as combined tetanus, diphtheria, and pertussis (although maternal tetanus vaccination is primarily to protect neonates from disease), and influenza vaccines. There are also a number of vaccine candidates currently under investigation that could potentially be licensed for the principal purpose of protecting the fetus and infant from infection, including vaccines that protect against cytomegalovirus (CMV), respiratory syncytial virus (RSV), and Group B Streptococcus (GBS).
The Assessment of Vaccine Safety in Pregnancy
The first documented vaccine trial in pregnant women was conducted in Papua New Guinea in 1961, during which administration of 2 or more doses of fluid formalinized tetanus toxoid vaccine during pregnancy was shown to be protective against neonatal tetanus.6 At the time, the United States Food and Drug Administration (FDA) guidelines excluded pregnant women from all drug and vaccine trials, and following the thalidomide tragedy in the 1950s to 60s, this exclusion was expanded to all women of childbearing potential.7 This decision was subsequently reversed by the FDA in 1993 after it was deemed that exclusion of this group of women had led to a substantial lack of safety data for a number of drugs in women of childbearing age.7 Even so, pregnant and lactating women still remain underrepresented among vaccine trial participants.
Generally, vaccines that are considered safe for administration during pregnancy include killed or inactivated virus vaccines, protein subunit vaccines, toxoid-containing vaccines, and conjugate vaccines (which include protein–toxoid, peptide–protein and protein–protein conjugated vaccines). Vaccines that contain live attenuated viruses are generally not considered safe because of the theoretical risk of congenital infection and the potential increased risk of miscarriage. Recent data from a meta-analysis conducted by Laris-González et al, however, did not identify any evidence of increased adverse pregnancy outcomes relating to the use of live vaccines during pregnancy other than for smallpox vaccines (although the quality of evidence included was low).8 In certain limited circumstances, a risk-benefit approach may be reasonably taken as to the appropriateness of administering a live vaccine, particularly in situations where the risk posed to the mother is deemed to significantly outweigh the theoretical risks posed to the fetus (discussed in further detail later).
With the advent of novel vaccine platforms such as the messenger RNA (mRNA) and nonreplicating viral vector platforms used in the production of the COVID-19 vaccines, the assessment of vaccine safety in pregnancy has re-emerged as an area of high priority owing to the limited historic data supporting their use. The assessment of vaccine safety in pregnant women requires additional safeguards to ensure that the pregnancy and neonatal outcomes are appropriately monitored. Knowledge of the background rates of adverse pregnancy and neonatal outcomes among the study population is also needed for accurate causality assessments. This requirement may limit the researchers’ ability to conduct maternal vaccine trials in resource-limited settings where such data are not routinely reported.9 In the United States, the Vaccine Adverse Event Reporting System (VAERS) is used for postlicensure vaccine safety monitoring, in which data are collected on adverse events after vaccination, such as stillbirth, miscarriage, and birth defects.10 In a recent study by Moro et al, VAERS reports relating to pregnant women vaccinated between 2000 and 2014 identified only 50 major birth defects, and no unusual clusters of birth defects were seen among these reports.11
At present, vaccines undergo at least Phase 1 and 2 studies in nonpregnant women of childbearing potential before they become eligible for Phase 1 evaluation in pregnant women. In circumstances where the need for a vaccine is urgent, such as during disease outbreaks, this process can cause an undue delay in providing sufficient safety data to support the use of the vaccine in pregnant and lactating women. Both the Sierra Leone Trial to Introduce a Vaccine Against Ebola trial (ClinicalTrials.gov Identifier: NCT02378753), which evaluated the recombinant vesicular stomatitis virus–Zaire Ebola virus vaccine against Ebola, and the recently conducted COVID-19 vaccine trials did not initially include pregnant and lactating women.12 , 13 In both circumstances, the initial vaccine safety data in pregnancy were collected from pregnant women who either inadvertently or deliberately received the vaccine in/outside of trials, highlighting the need for a more coordinated approach to facilitate the earlier inclusion of pregnant women in these trials.12 , 14 Strategies that may enable their inclusion include the incorporation of developmental toxicology studies into the vaccine programs at an early time point and the early use of vaccine platforms that are already known to be safe in pregnancy.13
Increasing Vaccine Confidence Among Pregnant Women
Low rates of vaccine confidence among pregnant women remain a significant barrier to increasing vaccination coverage among pregnant women, with persistently low rates of vaccine uptake during pregnancy seen in the US and many countries worldwide.15 A systematic review by Kilich et al,16 which reviewed the factors that influenced vaccine uptake in pregnant women, found that the main determinants were awareness of the vaccine, disease severity and susceptibility, vaccine benefits, side effects and risk of harm during pregnancy, history of previous vaccination, and recommendation from healthcare professionals. It is important that pregnant women are proactively offered the vaccine by their healthcare providers and are given ample time and opportunity to communicate any concerns they may have, while also being provided with sufficient information to help them make an informed decision. It is also important that healthcare professionals are provided with the training needed to be able to effectively counsel and support pregnant women through this decision-making process.17 The additional solutions recommended for increasing vaccine uptake among pregnant women include increased healthcare provider endorsement of the vaccine; increased healthcare provider and patient education as to the benefits of vaccination; improved regulatory processes, including more transparent labeling of vaccines; and multichannel approaches that include community education programs and use of media to promote the vaccine.18 Marginalized members of society, such as members of migrant communities, have also been identified as having lower rates of vaccine uptake. Thus, it is also imperative that barriers to accessing healthcare are addressed for these women to improve coverage rates among this particularly vulnerable cohort.19 Targeted messaging that specifically highlights the benefits of vaccination during pregnancy may help women to feel more confident in their decision to take up these offers of vaccination.
Vaccines routinely recommended during pregnancy
The following vaccines are routinely recommended for administration during pregnancy by both international and national health organizations. A summary of the recommended dosing schedules and contraindications is shown in Table 1 . A more detailed summary of COVID-19 vaccines available internationally is shown in Table 2 .
Table 1.
Summary of vaccines recommended for administration during pregnancy in the United States
| Vaccine Brand name (manufacturer) |
Number of doses recommended | Recommended dosing schedule (gestation) | Contraindications |
|---|---|---|---|
| Influenza AFLURIA (Seqirus Pty. Ltd), Agriflu (Seqirus Inc), FLUAD (Seqirus Inc), Fluarix (GSK), Flublok (Protein Sciences Corporation), Flucelvax (Seqirus Inc), FluLaval (ID Biomedical Corporation of Quebec), FluMist, Fluvirin (Sequris Vaccines Ltd), Fluzone (Sanofi Pasteur) |
One dose | Vaccine can be administered during any trimester. Administration before the start of flu season is recommended | Contraindicated in individuals with a history of severe allergic reaction (eg, anaphylaxis) or life-threatening reaction to a previous dose of an influenza vaccine |
| Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis (Tdap) Adcel (Sanofi Pasteur), Boostrix (GSK) |
One dose | Between 27 and 36 weeks’ gestation (can be given earlier if indicated, eg, for wound management or pertussis outbreak) If no history of previous vaccination and dose not administered during pregnancy, give dose immediately postpartum |
Contraindicated in individuals who have had a severe allergic reaction (eg, anaphylaxis) after a previous dose of a Tdap vaccine or who have a severe allergy to any vaccine component |
Adapted from Centers for Disease Control and Prevention guidelines.20
CDC, Centers for Disease Control and Prevention.
Etti. Maternal vaccination. Am J Obstet Gynecol 2022.
Table 2.
Summary of COVID-19 vaccines and evidence of safety and recommendations for use in pregnancy
| Vaccine platform | Commercial developer (candidate name) | Mechanism of action | Assessment of safety in pregnancy | Recommendations for use during pregnancy |
|---|---|---|---|---|
| mRNA | Pfizer/BioNTech (BNT162b2) | Nucleoside-modified mRNA expressed in lipid nanoparticles that encodes the spike protein for the SARS-COV-2 virus | Pfizer/BioNTech commenced a global Phase 3 study recruiting pregnant women in early 2021 | Initial safety data supports the safe use of mRNA vaccines in pregnant women |
| Moderna (mRNA-1237) | Nucleoside-modified mRNA encoding the pre-fusion stabilized spike (S) protein and the S1–S2 cleavage site encapsulated within a lipid nanoparticle | Real-world data from >90,000 women have not identified any safety signals22 | ||
| Nonreplicating viral vector | Oxford-AstraZeneca (AZD1222) | Modified chimpanzee adenovirus (replication deficient) containing the gene encoding the spike (S) protein | Pregnancies that occurred in clinical trials were recorded and followed up until 3 months after birth. Compared with women who received the control vaccine, there was no increased risk of miscarriage and no instances of stillbirth.23 | No previous studies among pregnant women. However, adenovirus-vectored Zika vaccine studies in pregnant mice did not identify any safety signals |
| Janssen (Ad26.COV2.S) | Recombinant, replication-incompetent human adenovirus type 26 that encodes the full length of the stabilized conformation of the spike (S) protein | |||
| Sputnik V (Gam-COVID-Vac) | Combined recombinant adenovirus-based vaccine (rAd5 and rAd26), both containing the gene encoding the full-length spike (S) protein | |||
| Protein subunit | Novavax (NVX-Cov2373) | Full length recombinant spike (S) protein nanoparticle administered with a saponin-based adjuvant (Matrix-M) | No direct safety data available | Recombinant vaccines are generally considered safe for use during pregnancy Safety of saponin-based adjuvant in pregnancy unknown |
| Inactivated whole virus | Sinovac (CoronaVac) | Inactivated whole virus particle containing aluminum hydroxide adjuvant | No direct safety data available | Inactivated vaccines generally considered safe for use during pregnancy. |
| Sinopharm (BBIBP-CorV) | Inactivated whole virus particle containing aluminum hydroxide adjuvant | Aluminum hydroxide (used in human papillomavirus vaccine) and CpG 1018 (used in hepatitis B virus vaccine adjuvants) both considered safe for use during pregnancy | ||
| Valneva (VLA2001) | Inactivated whole virus particle containing aluminum hydroxide and CpG 1018 adjuvants | Safety of the Alhydroxiquim-II adjuvant unknown in pregnancy | ||
| Bharat Biotech (BBV152) | Inactivated whole virus particle containing Alhydroxyquim-II adjuvant |
Adapted from Kalafat et al.21
mRNA, messenger RNA.
Etti. Maternal vaccination. Am J Obstet Gynecol 2022.
Influenza
Current recommendation:
-
•
Centers for Disease Control and Prevention (CDC): One dose of the seasonal influenza vaccine recommended during any trimester of pregnancy24
-
•
World Health Organization (WHO): Pregnant women should be prioritized to receive the seasonal influenza vaccine (1 dose). The influenza vaccine should be made available to pregnant women all year round.25
Vaccine coverage among pregnant women:
Many studies have shown that pregnant women are at a greater risk of severe disease and death from seasonal influenza than nonpregnant women.27, 28, 29 Similar outcomes were seen during the 2009 Influenza A pandemic, where pregnant women were 7.2% more likely to be hospitalized than nonpregnant women and were also found to have a disproportionally high risk of mortality.30 , 31 One recently conducted prospective cohort study also found that pregnant women who were infected with influenza during pregnancy were more likely to experience adverse pregnancy outcomes, including late pregnancy loss (adjusted hazard ratio, 10.7; 95% confidence interval [CI], 4.3–27.0) and a reduction in the birthweight of their infants, compared with women who were not infected.27
In light of the increased risks to pregnant women, since 2012, the WHO has advised that pregnant women should be prioritized to receive the seasonal influenza vaccine all year round.25 , 32 The inactivated virus vaccine, containing either 3 (trivalent influenza vaccine) or 4 (quadrivalent influenza vaccine) strains of the influenza virus, is recommended for administration during pregnancy. The live attenuated influenza vaccine, which is administered intranasally, is contraindicated during pregnancy because of the theoretical risk of placental transmission of the virus to the fetus.
There is no current consensus on the optimal gestational timing of vaccine administration. In the United States, pregnant women are advised to receive their vaccination in anticipation of the influenza season.33 One systematic review and meta-analysis found that the rate of seroconversion did not differ significantly among pregnant women who received their vaccines during different trimesters, although the geometric mean titers of neutralizing antibodies against influenza in the cord blood were found to be 1.44 (95% CI, 0.95–2.44) times higher among the women who were vaccinated during the third trimester than those vaccinated in the first trimester of pregnancy.34 However, there is evidence that the risk of fetal death and adverse birth outcomes is greatest for women who are infected during their first trimester of pregnancy,35 strengthening the rationale for vaccinating them earlier in pregnancy.
In addition to the placental transfer of maternal IgG antibodies, infants may also receive protection from influenza through secretory IgA antibodies present in the vaccinated mother’s breast milk. In a study conducted by Schlaudecker et al,36 sustained high levels of influenza-specific IgA antibodies were found in the breast milk of women vaccinated against influenza during pregnancy for up to 6 months after birth.
Tetanus
Current recommendation:
-
•
CDC: One dose (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis [Tdap]) recommended between 27 and 36 weeks’ gestation
-
•WHO:
-
○If previously received 1 to 4 doses of tetanus toxoid with tetanus and diphtheria (TT/Td), give 1 dose at least 2 weeks before delivery
-
○If not previously received a dose of TT/Td or vaccination status is unknown, give 2 doses of TT/Td at least 4 weeks apart, with the second dose given at least 2 weeks before delivery37
-
○
Vaccine coverage among pregnant women:
Maternal and neonatal tetanus is now largely not seen in high-income nations, but high mortality rates from the disease are still evident among women and children in many low- and middle-income countries.39 In response to this, the WHO launched the Maternal and Neonatal Tetanus Elimination initiative in 1999 in partnership with the United Nations Children’s Fund and the United Nations Population Fund.40 Since this time, maternal and neonatal tetanus has been eliminated in 47 out of 59 “at-risk” countries through a combination of increased maternal and neonatal vaccine coverage, and improved hygiene during delivery (Figure 3 ).39 , 40
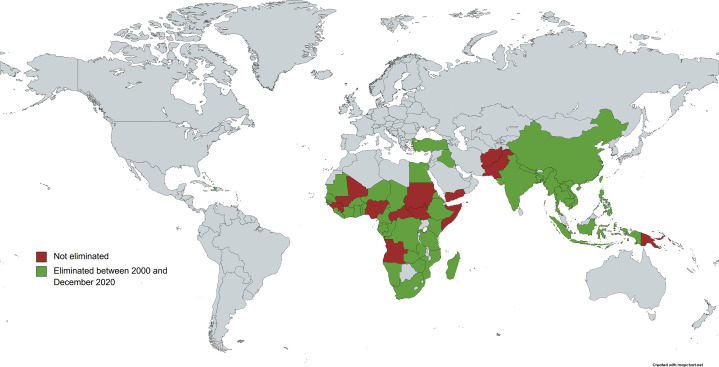
Figure 3.
Global elimination status of maternal and neonatal tetanus
As of December 2020, 12 out of 59 “at-risk” countries identified by the WHO in 2000 had not yet eliminated the disease.40 Figure reproduced with permission from the World Health Organization.
Countries shaded in green represents maternal and neonatal tetanus eliminated between 2000 and December 2020
Countries shaded in red represents maternal and neonatal tetanus not eliminated.
WHO, World Health Organization.
Etti. Maternal vaccination. Am J Obstet Gynecol 2022.
There are 4 tetanus toxoid-containing vaccines that are considered safe for use in pregnancy: tetanus toxoid (TT), tetanus toxoid and reduced-dose diphtheria toxoid (Td), (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap), and Tdap in combination with the inactivated polio vaccine (Tdap/IPV). TT was previously widely used; however, the WHO now recommends that a tetanus-diphtheria combination vaccine should be administered instead of TT to provide early childhood protection against diphtheria.41 The WHO recommends that a total of 5 doses of TT or Td are required to provide protection throughout the childbearing years.37 If the pregnant woman has not previously received any doses of TT, Td, or Tdap, or her vaccination history is uncertain, additional doses are recommended after pregnancy to ensure full protection (Table 3 ).37 In high-income nations where neonatal tetanus has been eliminated, Tdap or Tdap/IPV is administered during pregnancy with the primary purpose of preventing infant pertussis.42
Table 3.
Tetanus toxoid vaccination schedule for pregnant women and women of childbearing age with no or uncertain previous exposure to tetanus toxoid; tetanus toxoid and reduced-dose diphtheria toxoid; or diptheria, pertussis and tetanus37
| Dose of TT or Td (according to card or history) | When to give | Expected duration of protection |
|---|---|---|
| 1 | At first contact or as early as possible in pregnancy | None |
| 2 | At least 4 wk after TT1 | 1–3 y |
| 3 | At least 6 mo after TT2 or during subsequent pregnancy | At least 5 y |
| 4 | At least 1 y after TT3 or during subsequent pregnancy | At least 10 y |
| 5 | At least 1 y after TT4 or during subsequent pregnancy | For all childbearing age years or possibly longer |
Table reproduced with permission from the World Health Organization.
TT, tetanus toxoid, Td, tetanus toxoid and reduced-dose diphtheria toxoid.
Etti. Maternal vaccination. Am J Obstet Gynecol 2022.
Pertussis
Current recommendation:
-
•
CDC: One dose (Tdap) recommended between 27 and 36 weeks’ gestation
-
•
WHO: National programs may consider vaccination of pregnant women with a pertussis-containing vaccine as a strategy additional to routine primary infant pertussis vaccination in countries or settings with high or increasing infant morbidity or mortality from pertussis43
Vaccine coverage among pregnant women:
-
•
United States: Tdap vaccine coverage 56.6%26
-
•
Worldwide: data not available
Pertussis is a highly infectious respiratory disease that can cause serious illness in young infants. Pertussis vaccines have been available since the 1950s, and their widespread use significantly reduced the incidence of pertussis disease globally. There has been a resurgence of pertussis cases in many countries, including in those with good vaccine coverage, with high rates of the disease in infants. In the United States, the cases of pertussis rose from 7857 in 2000 to over 48,000 cases in 2012.44 In 2005, “cocooning” was recommended by the Advisory Committee on Immunization Practices in response to the increasing number of cases, whereby the close contacts of infants were advised to get vaccinated against pertussis. However, this advice was later revised after it was found that cocooning was poorly effective.45 , 46 The WHO recommends vaccination of pregnant women as a more cost-effective means of prevention of pertussis in infants than cocooning.47
Many countries worldwide have introduced pertussis vaccination for pregnant women to protect infants from the disease. Although these programs have been shown to be effective in preventing severe pertussis disease in infants,48, 49, 50, 51 there is uncertainty about the best time during pregnancy to offer vaccination to provide optimal protection. Some investigators have suggested that later administration is preferable to coincide with maximal antibody transfer, whereas others have reported higher antibody titers at birth in babies born to mothers who were vaccinated earlier in pregnancy.52, 53, 54 Studies evaluating the safety of the Tdap vaccine have not identified any serious adverse events associated with its use during pregnancy.55 , 56
Pertussis vaccination in pregnancy results in higher antibody levels in the infant at birth, and this persists for at least 2–3 months. In addition, high levels of pertussis-specific IgA antibodies have been detected in the colostrum of women vaccinated during pregnancy and are detectable in breast milk for up to 8 weeks postpartum.57 The increased serum levels in infants born to vaccinated mothers may lead to a reduced initial response to the infant’s own vaccinations against pertussis and diphtheria,58, 59, 61 though this reduction may not have any clinical implications, and the levels are generally restored following booster vaccinations.60 , 62
COVID-19
Current recommendation:
-
•
CDC: COVID-19 vaccination is recommended for all people aged ≥12 years, including people who are pregnant, breastfeeding, trying to get pregnant currently, or might become pregnant in the future63
-
•
WHO: the use of the COVID-19 vaccine in pregnant women is recommended when the benefits of vaccination to the pregnant woman outweigh the potential risks. To help pregnant women make this assessment, they should be provided with information about the risks of COVID-19 in pregnancy, the likely benefits of vaccination in the local epidemiologic context, and the current limitations of safety data in pregnant women. WHO does not recommend pregnancy testing before vaccination. It also does not recommend delaying pregnancy or terminating it because of vaccination.64 , 65
Vaccine coverage among pregnant women:
-
•
United States: 31%15
-
•
Worldwide: data not available
Data from many countries have identified pregnant women as being at a greater risk of severe disease and death from SARS-CoV-2 infection than nonpregnant women.66, 67, 68, 69, 70 In addition, COVID-19 in pregnancy is associated with an increased risk of adverse pregnancy outcomes.66 , 68 , 71 One large population-based cohort study in England found that among pregnant women who had SARS-CoV-2 infection at the time of delivery, there was a greater risk of preeclampsia or eclampsia (adjusted odds ratio [aOR], 1.57; 95% CI, 1.44–1.72), preterm delivery (aOR, 2.17; 95% CI, 1.96–2.42) and fetal death (aOR, 2.21; 95% CI, 1.58–3.11).72
Among the COVID-19 vaccines that have been licensed for use internationally, there are 4 main vaccine platforms that have been employed (Table 2). On December 9, 2020, the Pfizer/BioNTech mRNA vaccine was granted emergency use authorization (EUA) by the FDA after the Phase 3 study involving 43,000 nonpregnant participants demonstrated 95.0% efficacy against COVID-1914 , 73 and was granted full FDA approval on August 23, 2021.74 The COVID-19 vaccines manufactured by Moderna (mRNA-1237) and Janssen (Ad26.COV2.S) were granted EUA by the FDA on December 18, 2020 and February 27, 2021, respectively.75 , 76 Given the initial lack of safety data in pregnancy, a risk-based approach to vaccination was initially implemented, and clinicians in countries such as the United Kingdom and the United States were advised to recommend vaccination for “clinically vulnerable” women following an assessment of their exposure risk and clinical risk factors for severe disease.77 In April 2021, the CDC announced that pregnant women who are eligible for the COVID-19 vaccine could receive the vaccines manufactured by Pfizer/BioNTech and Moderna, after real-world data from 90,000 pregnant women collected through the V-safe COVID-19 vaccine pregnancy registry did not identify any safety signals.78 , 22
There are currently no data to guide recommendations for vaccine administration at a particular gestational age, although in practice, many women receive the vaccine during the second or third trimester, as they may wish to avoid any theoretical concerns around vaccination in the first trimester when organogenesis occurs.79 Recent studies conducted in the United States and Israel have demonstrated placental transfer of vaccine-specific anti-SARS-CoV-2 IgG antibodies, and anti-SARS-CoV-2 IgA and IgG antibodies have also been detected in the breast milk of lactating women who were vaccinated during pregnancy for up to 6 weeks after the first vaccine dose.80, 81, 82, 83 As serocorrelates of disease protection have not yet been defined, the antibody titers required to confer protection against disease in a pregnant woman or in the neonate are not known. Additional data are needed to determine the benefit of maternal vaccination for the developing fetus and infant (which may in turn provide guidance as to the optimal timing of vaccination) and also to determine the long-term safety of these novel vaccine technologies for offspring born to the women vaccinated during pregnancy. In February 2021, Pfizer/BioNTech began global recruitment to their Phase 2 and 3 trials (ClinicalTrials.gov Identifier: NCT04754594) evaluating the safety, tolerability, and immunogenicity of their COVID-19 vaccine among pregnant women between 27 and 34 weeks’ gestation, with trial completion expected in July 2022.84 Another Phase 2 trial has commenced in the United Kingdom, in which the optimal schedule of vaccination for pregnant women is being assessed (https://doi.org/10.1186/ISRCTN15279830).
Vaccines safe for use in pregnancy under special conditions
Apart from vaccines in routine use in pregnancy, some vaccinations can be used in specific circumstances; for example, in the context of an outbreak, before traveling, or after exposure to an infection. We have summarized the safety considerations and recommendations for use for this group of vaccines below.
Commonly used
-
1.
Hepatitis B
Vaccine platform: Recombinant subunit of the surface antigen protein
Safety considerations and recommendations for use:
There is no evidence that administration of the hepatitis B virus (HBV) vaccine in pregnancy prevents infant infection.85 Hepatitis B vaccination in pregnancy is not associated with an increase in adverse pregnancy outcomes.86 The CDC recommends that any pregnant patient who is at high risk of contracting HBV or who would like to receive the HBV vaccine can be offered the vaccine during pregnancy.20
-
2.
Neisseria meningitidis (meningococcal)
Vaccine platform: Polysaccharide and conjugate vaccines
Safety considerations and recommendations for use:
Meningococcal polysaccharide vaccines are safe,87, 88, 89, 90, 91 immunogenic, and result in higher antibody concentrations in the infant.87, 88, 89, 90, 91
Meningococcal conjugate vaccines have not been associated with any safety concerns in pregnancy.92, 93, 94 There is no evidence about their immunogenicity or effectiveness when given in pregnancy.
Vaccination can be recommended if a woman is at a high risk of meningococcal disease or in the context of an outbreak.
-
3.
Polio
Vaccine platform: Inactivated virus, live attenuated (oral)
Safety considerations and recommendations for use: The inactivated virus vaccine (IPV) is routinely offered to all pregnant women in the United Kingdom and New Zealand (in combination with the Tdap vaccine).95 , 96 The CDC does not recommend its routine administration to women who are not at an increased risk of exposure to the disease.97 The live attenuated preparation is contraindicated for use in pregnancy, although no adverse birth outcomes have been reported in women who received the oral polio vaccine during pregnancy.98
Less commonly used
-
1.
Anthrax
Vaccine platform(s): Recombinant protective antigen
Safety considerations and recommendations for use: No association has been shown between inadvertent anthrax vaccination in pregnancy and the risk of birth defects.99 , 100 Because of the severity of anthrax infection, it is recommended that pregnant women should receive the same postexposure prophylaxis as nonpregnant adults, including vaccination. If women are at risk of inhalational anthrax, they should receive anthrax vaccine regardless of gestation.101
-
2.
Cholera
Vaccine platform(s): Inactivated bacterium (oral vaccine); live attenuated
Safety considerations and recommendations for use:
The inactivated vaccine is theoretically safe, as bacteria within the vaccine are killed and cannot replicate and the vaccine antigens act locally on gastrointestinal mucosa and are unlikely to cause systemic toxicity. No increase in pregnancy adverse outcomes in those women who inadvertently received cholera vaccination in pregnancy have been reported in 3 retrospective studies which included nearly 3000 women in 3 countries,102, 103, 104 and a further observational study showed no increase in risk of pregnancy loss or of neonatal death.105 The WHO recommends that pregnant and lactating women are included in cholera vaccination campaigns as there is high potential benefit and minimal potential risk.47 The inactivated vaccine should also be considered on a case-by-case basis for women who are at high risk for disease. The live attenuated preparation is contraindicated for use in pregnancy.102, 103, 104
-
3.
Coxiella burnetii (Q fever)
Vaccine platform(s): Inactivated bacterium
Safety considerations and recommendations for use: There are no studies of Q fever vaccines in pregnancy and no official recommendations about their use.
-
4.
Haemophilus influenzae type b (Hib)
Vaccine platform(s): Polysaccharide and conjugate vaccines
Safety considerations and recommendations for use:
Both vaccine platforms are safe, immunogenic and result in increased antibody concentrations in the infant when administered in pregnancy, although conjugate vaccines are preferred because of the higher infant antibody concentrations at birth and at 2 months of age.106 , 107 There is no evidence of effectiveness in reducing disease incidence in infants.108 Hib vaccine could be used in pregnancy if considered necessary, however control of invasive Hib disease in many countries is extremely good and thus the need for Hib vaccination in pregnancy is likely to be low.
-
5.
Hepatitis A (HAV)
Vaccine platform(s): Inactivated virus; live attenuated
Safety considerations and recommendations for use:
There is no evidence of an increase in adverse pregnancy outcomes following inactivated hepatitis A vaccination in pregnancy. The inactivated virus vaccine can be used after consideration of the likely risks of exposure.109 , 110 The live attenuated preparation is contraindicated for use in pregnancy.
-
6.
Japanese encephalitis virus
Vaccine platform(s): Inactivated virus; live attenuated
Safety considerations and recommendations for use:
There is no evidence about the use of the inactivated Japanese encephalitis vaccine in pregnancy. The inactivated vaccine may be considered if traveling to an endemic area where one is likely to experience significant exposure. The live attenuated preparation is contraindicated for use in pregnancy.
-
7.
Rabies
Vaccine platform(s): Inactivated virus
Safety considerations and recommendations for use:
Postexposure prophylaxis: There is no evidence of an increased risk of adverse pregnancy outcome following the postexposure administration of the rabies vaccine when compared with the background rate of adverse outcomes.111, 112, 113, 114, 115, 116, 117, 118
Preexposure prophylaxis: Although studies have focused on the administration of the vaccine following exposure, the safety of the vaccine demonstrated in these studies would support its use before exposure for a pregnant woman at high risk.
Given the high case fatality rate for rabies, pregnancy should not be considered a contraindication to postexposure prophylaxis and may be considered for preexposure prophylaxis for women at risk.
-
9.
Streptococcus pneumoniae (pneumococcal)
Vaccine platform(s): Polysaccharide and conjugate vaccines
Safety considerations and recommendations for use:
Polysaccharide vaccines are safe119 and increase antipolysaccharide antibodies in infants,120, 121, 122, 123, 124, 125, 126, 127 though there is little evidence that this affects the colonization rates or disease incidence in infants born to vaccinated mothers.128 , 129
There is limited evidence for the use of conjugate vaccines in pregnancy; the only published study showed that infants of vaccinated mothers had an increased incidence of the primary outcome (acute otitis media).130
Pneumococcal vaccinations can be used in pregnancy if protection of the woman is considered necessary.
-
10.
Tick-borne encephalitis virus
Vaccine platform(s): Inactivated virus
Safety considerations and recommendations for use:
Theoretically, there are no contraindications for the use of this vaccine in pregnancy. However, there are no studies of tick-borne encephalitis virus vaccines in pregnant women and no official recommendations for their use.
-
11.
Typhoid
Vaccine platform(s): Oral live attenuated; polysaccharide
Safety considerations and recommendations for use:
The safety of the polysaccharide vaccine has not been determined, but the theoretical risk is low. Therefore, it may be considered when the benefits are likely to outweigh the risks. The live attenuated preparation is contraindicated for use in pregnancy.
-
12.
Yellow fever
Vaccine platform(s): Live attenuated
Safety considerations and recommendations for use:
There is only a live attenuated vaccine available for the prevention of yellow fever. Live vaccines are usually contraindicated in pregnancy. However, there is some evidence that yellow fever vaccination in pregnancy is not associated with an increased incidence of adverse pregnancy outcomes, though congenital infection is possible.110 , 131, 132, 133 Use of the live vaccine can be considered if it is thought that the risks of infection outweigh the possible risks of vaccination.134 If the risks of vaccination are considered to outweigh the risks of yellow fever, but travel is required to an area that requires vaccination, a medical waiver can be issued.
Vaccines currently under investigation
-
1.
Group B Streptococcus
GBS is one of the leading causes of neonatal sepsis and meningitis globally.135 Maternal rectovaginal GBS colonization has also been associated with an increased risk of preterm delivery and stillbirth. Thus, there is a need to protect the fetus and provide passive immunity to protect infants after birth.136
Six capsular polysaccharide serotypes of GBS (Ia, Ib, II, III, IV, and V) cause approximately 98% of invasive GBS disease in neonates, with serotype III causing the greatest proportion of invasive disease.137 , 138 In 1988, Baker and Kasper first demonstrated the feasibility of maternal GBS vaccination, though the initial observations were of poor immunogenicity of their monovalent polysaccharide-based GBS vaccine, which was targeted against serotype III.139 More promising results have been seen with protein-conjugated capsular polysaccharide GBS vaccines, though the trivalent CRM197-conjugated capsular polysaccharide GBS vaccine developed by Novartis (targeted against serotypes Ia, Ib, and III) did not progress past Phase 1/2 studies (ClinicalTrials.gov Identifier: NCT02046148). A recent Phase 1/2 trial conducted by Absalon et al (ClinicalTrials.gov Identifier: NCT03170609) demonstrated the safety and immunogenicity of Pfizer’s novel hexavalent conjugate vaccine (GBS6) in nonpregnant adults, with the GBS serotype-specific geometric mean antibody concentrations remaining substantially elevated among the vaccinated groups 6 months after vaccination (between 10- and 56-fold higher than the placebo group).140 Pfizer has subsequently commenced the recruitment of pregnant women to their Phase 1/2 trial (ClinicalTrials.gov Identifier: NCT03765073).
In June 2020, Minervax started Phase 2 trials evaluating their recombinant protein-based vaccine (GBS-NN), which is based on the highly immunogenic N-terminals of the AlphaC and Rib GBS surface proteins (ClinicalTrials.gov Identifier: NCT04596878).141 This study will evaluate the safety and immunogenicity of the vaccine in pregnant women with and without HIV, which will be of particular value in sub-Saharan Africa where the rates of invasive GBS disease in neonates and HIV among women of reproductive age are high.142 , 143
-
2.
Cytomegalovirus
CMV is a very common infection that usually causes only a mild, self-limiting illness in healthy individuals but can cause more serious illness in those with reduced immunity; it is an important cause of congenital infection if women are infected during pregnancy. Congenital CMV is the most common cause of congenital deafness globally, and the development of a vaccine is a priority, which was recognized by the US National Academy of Medicine in 2000.144
Congenital infection can occur in women who have never had CMV before and are infected during pregnancy (primary infection); it can also occur in women who were infected with CMV before pregnancy and either have reactivation of infection or are infected with a different strain in pregnancy (secondary infection), though the risk of congenital infection in infants is the greatest in those with primary infection.145 These different modes of infection have made vaccine development complex, as has our limited understanding of the exact mechanisms by which maternal immunity protects the fetus. It seems that antibodies are a necessary mediator of protection for seronegative women. However, T-cell responses also play a vital role in suppressing viral reactivation in women who are seropositive.146 Therefore, a vaccine that induces both antibody and cellular responses is likely to be needed. Breast milk can also transfer maternal immune cells to the infant. Leukocyte populations in breast milk are distinct from those found in maternal blood, with an enrichment of CD8+ T cells, predominantly of the effector memory subtype.147 The exact function of these cells in infants is not yet known, but evidence from animal models suggests that they may be compensating for the infant’s immature adaptive immune system as they localize in the Peyer’s patches; their cytolytic and inflammatory activity is 4 times higher than that of the infant’s own T cells.148 There is also evidence that these breast milk CD8+ T cells may be could confer passive cellular immunity even after lysis in the infant gut.149
CMV vaccine development has been ongoing since the 1970s. Initial efforts were focused on live attenuated strains, the most extensively studied of which was the Towne strain. This was well-tolerated in nonpregnant adults but provided only incomplete protection.150 Following this, glycoprotein B (gB)—a surface protein of CMV—was identified, and vaccines based on it were shown to produce a good neutralizing antibody response with up to 50% efficacy against the disease. However, the antibody response was not persistent.151 , 152 Subsequently, a pentameric complex was discovered, which could produce higher titers of neutralizing antibodies than gB vaccines and which has been shown to provide protection against placental transmission.151 CMV vaccines that are currently in advanced stages of development include a replication-defective pentameric vaccine, an adjuvanted gB-based vaccine, viral vector vaccines, RNA vaccines, and a DNA plasmid vaccine.153 Moderna completed enrolment into their Phase 2 study investigating the safety and immunogenicity of their CMV mRNA vaccine (mRNA-1647) in men and women of childbearing age in March 2020 (ClinicalTrials.gov Identifier: NCT04232280). Enrolment into the Phase 3 study is expected to commence in late 2021.
-
3.
Respiratory syncytial virus
RSV is a major cause of acute lower respiratory tract infection in infants and young children worldwide.154 Infants are particularly vulnerable to RSV infection during early life; one population-based study found that infants aged <2 months old accounted for 44% of RSV hospitalizations, and very preterm infants (born at <30 weeks’ gestation) were 3 times more likely to be hospitalized than infants born at term.155 The treatment of RSV infection is mainly supportive, though palivizumab (Synagis, Sobi), a humanized monoclonal antibody that targets the antigenic site of the fusion (F) glycoprotein of RSV, has been shown to be effective in reducing the incidence of hospitalization among high-risk children aged <24 months.156 , 157
In the 1960s, a formalin-inactivated RSV vaccine was trialed in infants and toddlers. However, increased rates of hospitalization and deaths because of RSV were seen that winter among these children due, in part, to the nonprotective, low-avidity IgG response elicited by the vaccine.156 Maternal vaccination is believed to be a safer means of conferring immunity in infants against the virus, and although a number of maternal RSV vaccine candidates have been developed, none have yet been licensed for use. The efficacy of palivizumab against severe RSV infection has identified the F glycoprotein as a promising vaccine target. However, no vaccines have yet shown sufficient efficacy in disease reduction in Phase 3 trials.158 One recent Phase 3 trial investigating the efficacy of the Novavax recombinant RSV fusion nanoparticle vaccine between 28 and 36 weeks’ gestation (NCT02624947) did not show it to be sufficiently efficacious in preventing RSV-associated, medically-significant lower respiratory tract infections during the first 90 days of life (efficacy 39%; 97.52% CI, −1.0 to 63.7; prespecified lower boundary of 97.52% CI ≥30%). However, fewer infants within the study group were hospitalized because of RSV-associated lower respiratory tract infections than in the placebo group (2.1% vs 3.5%, vaccine efficacy 44%; 95% CI, 19.6% to 61.5%).159 Animal models and observational human studies have more recently demonstrated the superiority of the prefusion form of the F glycoprotein in stimulating the production of neutralizing antibodies against RSV.160 , 161 In 2020, Pfizer (ClinicalTrials.gov Identifier: NCT04424316) and GlaxoSmithKline (ClinicalTrials.gov Identifier: NCT04605159) both commenced Phase 3 studies of their respective recombinant subunit prefusion RSV F antigen vaccine candidates, with completion of both studies expected between 2023 and 2024.
Vaccines contraindicated during pregnancy
The vaccines that are not recommended or contraindicated during pregnancy are summarized in Table 4 . The inadvertent administration of these vaccines during pregnancy, for example, before the woman realizes she is pregnant, is not an indication for termination of pregnancy. However, there should be counseling regarding the potential risks to the fetus.20
Table 4.
Vaccines contraindicated during pregnancy
| Vaccine (platform) | Reason for contraindication | Safety considerations |
|---|---|---|
| BCG (live attenuated virus) | Contains live culture preparation of the BCG strain of Mycobacterium bovis | No harmful effects have been observed in pregnant women. However, safety in pregnancy has not been formally evaluated.162 |
| Human papilloma virus (recombinant virus-like particle) | No safety data available to support use in pregnancy. Not recommended by the CDC for administration during pregnancy. | No evidence of increased risk of adverse pregnancy or fetal outcomes following administration during pregnancy.163,164 If inadvertent administration during pregnancy, delay remaining doses until after pregnancy. |
| Measles, mumps, and rubella (live attenuated virus) | Contains live attenuated mumps, measles, and rubella viruses | No evidence of increased risk of adverse pregnancy or fetal outcomes (including congenital rubella syndrome) following administration during pregnancy.98 Pregnancy testing is not recommended before vaccine administration of vaccine. However, recipients are advised not to become pregnant for at least 28 days after vaccine dose.20,47 |
| Varicella (live attenuated virus) | Contains live attenuated varicella-zoster virus. | Data from Merck/CDC Pregnancy Registry have not identified any increased risk of congenital varicella syndrome.20,165 |
| Zoster (recombinant glycoprotein) | No safety data available to support use in pregnancy. Not recommended by CDC for administration during pregnancy. | Data from Merck/CDC Pregnancy Registry has not identified any increased risk of congenital varicella syndrome.20 |
BCG, Bacillus Calmette-Guérin; CDC, Centers for Disease Control and Prevention.
Etti. Maternal vaccination. Am J Obstet Gynecol 2022.
Conclusion
Maternal vaccination is an effective yet underutilized means of infectious disease prevention for pregnant women and their infants. Pregnant women should be informed of the potential benefits of vaccination for themselves, their fetuses, and infants and should be proactively offered routinely recommended vaccines to allow timely administration before delivery of the infant. Sufficient time should be allowed to address any concerns women may have regarding the safety of these vaccine during pregnancy. In addition, healthcare providers should be provided with sufficient training to support pregnant women throughout the decision-making process. Currently, it is recommended that all pregnant women should be routinely offered influenza, tetanus, and pertussis-containing vaccines. Pregnant and lactating women, and also women who are intending to get pregnant, should now be routinely offered the COVID-19 vaccine in view of the mounting evidence of its safety.
There are still a number of vaccines under development that may be licensed for use in pregnancy within the next decade. Additional data are needed to determine the long-term safety of newly developed vaccine technologies that have not been previously evaluated in pregnancy, including RNA and nonreplicating viral vector vaccine platforms.
Footnotes
P.T.H. reports grant funding to his institution from vaccine manufacturers, including Pfizer, Novavax, and Minervax. The other authors report no conflict of interest.
The authors received no specific funding for this work.
Supplementary Data
References
- 1.Jones C., Heath P. Antenatal immunizations concepts and challenges. Hum Vaccin Immunother. 2014;10:2118–2122. doi: 10.4161/hv.29610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmeira P., Quinello C., Silveira-Lessa A.L., Zago C.A., Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht M., Arck P.C. Vertically transferred immunity in neonates: mothers, mechanisms and mediators. Front Immunol. 2020;11:555. doi: 10.3389/fimmu.2020.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Martino M. Dismantling the taboo against vaccines in pregnancy. Int J Mol Sci. 2016;17:894. doi: 10.3390/ijms17060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heath P.T., Jardine L.A. Neonatal infections: group B streptococcus. BMJ Clin Evid. 2014;2014:0323. [PMC free article] [PubMed] [Google Scholar]
- 6.Schofield F.D., Tucker V.M., Westbrook G.R. Neonatal tetanus in New Guinea. Effect of active immunization in pregnancy. Br Med J. 1961;2:785–789. doi: 10.1136/bmj.2.5255.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macklin R. Enrolling pregnant women in biomedical research. Lancet. 2010;375:632–633. doi: 10.1016/s0140-6736(10)60257-7. [DOI] [PubMed] [Google Scholar]
- 8.Laris-González A., Bernal-Serrano D., Jarde A., Kampmann B. Safety of administering live vaccines during pregnancy: a systematic review and meta-analysis of pregnancy outcomes. Vaccines (Basel) 2020;8:124. doi: 10.3390/vaccines8010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochhar S., Bonhoeffer J., Jones C.E., et al. Immunization in pregnancy clinical research in low- and middle-income countries - study design, regulatory and safety considerations. Vaccine. 2017;35:6575–6581. doi: 10.1016/j.vaccine.2017.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention, Food and Drug Administration, US Department of Health and Human Services Vaccine Adverse Event Reporting System (VAERS) 2021. https://vaers.hhs.gov/ Available at:
- 11.Moro P.L., Cragan J., Lewis P., Sukumaran L. Major birth defects after vaccination reported to the Vaccine Adverse Event Reporting System (VAERS), 1990 to 2014. Birth Defects Res. 2017;109:1057–1062. doi: 10.1002/bdr2.23622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Legardy-Williams J.K., Carter R.J., Goldstein S.T., et al. Pregnancy outcomes among women receiving RVSVΔ-Zebov-GP Ebola vaccine during the Sierra Leone trial to introduce a vaccine against Ebola. Emerg Infect Dis. 2020;26:541–548. doi: 10.3201/eid2603.191018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heath P.T., Le Doare K., Khalil A. Inclusion of pregnant women in COVID-19 vaccine development. Lancet Infect Dis. 2020;20:1007–1008. doi: 10.1016/S1473-3099(20)30638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Health Alert Network COVID-19 vaccination for pregnant people to prevent serious illness, deaths, and adverse pregnancy outcomes from COVID-19. 2021. https://emergency.cdc.gov/han/2021/han00453.asp Available at:
- 16.Kilich E., Dada S., Francis M.R., et al. Factors that influence vaccination decision-making among pregnant women: a systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson R.J., Paterson P., Jarrett C., Larson H.J. Understanding factors influencing vaccination acceptance during pregnancy globally: a literature review. Vaccine. 2015;33:6420–6429. doi: 10.1016/j.vaccine.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 18.Buchy P., Badur S., Kassianos G., Preiss S., Tam J.S. Vaccinating pregnant women against influenza needs to be a priority for all countries: an expert commentary. Int J Infect Dis. 2020;92:1–12. doi: 10.1016/j.ijid.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Adeyanju G.C., Engel E., Koch L., et al. Determinants of influenza vaccine hesitancy among pregnant women in Europe: a systematic review. Eur J Med Res. 2021;26:116. doi: 10.1186/s40001-021-00584-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Pregnancy guidelines and recommendations by vaccine. 2016. https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/guidelines.html#hpv Available at:
- 21.Kalafat E., O’Brien P., Heath P.T., et al. Benefits and potential harms of COVID-19 vaccination during pregnancy: evidence summary for patient counseling. Ultrasound Obstet Gynecol. 2021;57:681–686. doi: 10.1002/uog.23631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimabukuro T.T., Kim S.Y., Myers T.R., et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillson K., Clemens S.C., Madhi S.A., Voysey M., Pollard A.J., Minassian A.M. Oxford COVID Vaccine Trial Group. Fertility rates and birth outcomes after ChAdOx1 nCoV-19 (AZD1222) vaccination. Lancet. 2021;6;398:1683–1684. doi: 10.1016/S0140-6736(21)02282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention Influenza (flu) vaccine and pregnancy. 2019. https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/flu-vaccine-pregnancy.html Available at:
- 25.Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012;87:461–476. [PubMed] [Google Scholar]
- 26.Razzaghi H., Kahn K.E., Black C.L., et al. Influenza and Tdap vaccination coverage among pregnant women - United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1391–1397. doi: 10.15585/mmwr.mm6939a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawood F.S., Kittikraisak W., Patel A., et al. Incidence of influenza during pregnancy and association with pregnancy and perinatal outcomes in three middle-income countries: a multisite prospective longitudinal cohort study. Lancet Infect Dis. 2021;21:97–106. doi: 10.1016/S1473-3099(20)30592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mertz D., Lo C.K.F., Lytvyn L., Ortiz J.R., Loeb M., FLURISK-INVESTIGATORS Pregnancy as a risk factor for severe influenza infection: an individual participant data meta-analysis. BMC Infect Dis. 2019;19:683. doi: 10.1186/s12879-019-4318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vousden N., Bunch K., Knight M. UKOSS Influenza Co-Investigators Group. Incidence, risk factors and impact of seasonal influenza in pregnancy: a national cohort study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0244986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Creanga A.A., Johnson T.F., Graitcer S.B., et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115:717–726. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 31.Siston A.M., Rasmussen S.A., Honein M.A., et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. How to Implement Influenza Vaccination of Pregnant Women. 2017. Available at: https://www.who.int/publications-detail-redirect/WHO-IVB-16.06. Accessed November 28, 2021.
- 33.American College of Obstetricians and Gynecologists Influenza vaccination during pregnancy. 2018. https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/04/influenza-vaccination-during-pregnancy Available at:
- 34.Cuningham W., Geard N., Fielding J.E., et al. Optimal timing of influenza vaccine during pregnancy: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2019;13:438–452. doi: 10.1111/irv.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunnes N., Gjessing H.K., Bakken I.J., et al. Seasonal and pandemic influenza during pregnancy and risk of fetal death: a Norwegian registry-based cohort study. Eur J Epidemiol. 2020;35:371–379. doi: 10.1007/s10654-020-00600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlaudecker E.P., Steinhoff M.C., Omer S.B., et al. IgA and neutralizing antibodies to influenza A virus in human milk: a randomized trial of antenatal influenza immunization. PLoS One. 2013;8:e70867. doi: 10.1371/journal.pone.0070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization Maternal immunization against tetanus. 2002. https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/immunization_tetanus.pdf Available at:
- 38.Njuguna H.N., Yusuf N., Abid Raza A.A., Ahmed B., Tohme R.A. Progress toward maternal and neonatal tetanus elimination - worldwide, 2000-2018. MMWR Morb Mortal Wkly Rep. 2020;69:515–520. doi: 10.15585/mmwr.mm6917a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thwaites C.L., Beeching N.J., Newton C.R. Maternal and neonatal tetanus. Lancet. 2015;385:362–370. doi: 10.1016/S0140-6736(14)60236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization Progress towards global MNT elimination. 2021. https://www.who.int/initiatives/maternal-and-neonatal-tetanus-elimination-(mnte)/progress-towards-global-mnt-elimination Available at:
- 41.World Health Organization/United Nations Children’s Fund. Replacement of TT with Td vaccine for dual protection. 2018. Available at: http://www.who.int/immunization/programmes_systems/procurement/v3p/platform/WHO_DT_global_market_study.pdf.
- 42.Public Health England. Pertussis vaccination programme for pregnant women update: vaccine coverage in England, July to September 2020. Health Protection Report. 2020. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/941297/hpr2320_prtsss-vc.pdf. Accessed June 23, 2021.
- 43.World Health Organization Immunization, vaccines and Biologicals. 2021. https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/pertussis Available at:
- 44.Centers for Disease Control and Prevention Pertussis surveillance: cases by year. 2017. https://www.cdc.gov/pertussis/surv-reporting/cases-by-year.html Available at:
- 45.Blain A.E., Lewis M., Banerjee E., et al. An assessment of the cocooning strategy for preventing infant pertussis-United States, 2011. Clin Infect Dis. 2016;63(Suppl4):S221–S226. doi: 10.1093/cid/ciw528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention Vaccinate pregnant patients to protect against pertussis. 2017. https://www.cdc.gov/pertussis/pregnant/hcp/pregnant-patients.html Available at:
- 47.World Health Organization Recommendations for interrupted or delayed routine immunization—summary of WHO position papers. 2015. http://www.who.int/immunization/policy/Immunization%7B_%7Droutine%7B_%7Dtable3.pdf?ua=1$%5C$n Available at: Accessed November 11, 2021.
- 48.Vygen-Bonnet S., Hellenbrand W., Garbe E., et al. Safety and effectiveness of acellular pertussis vaccination during pregnancy: a systematic review. BMC Infect Dis. 2020;20:136. doi: 10.1186/s12879-020-4824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amirthalingam G., Andrews N., Campbell H., et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384:1521–1528. doi: 10.1016/S0140-6736(14)60686-3. [DOI] [PubMed] [Google Scholar]
- 50.Amirthalingam G., Campbell H., Ribeiro S., et al. Sustained effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clin Infect Dis. 2016;63(Suppl4):S236–S243. doi: 10.1093/cid/ciw559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Becker-Dreps S., Butler A.M., McGrath L.J., et al. Effectiveness of prenatal tetanus, diphtheria, acellular pertussis vaccination in the prevention of infant pertussis in the U.S. Am J Prev Med. 2018;55:159–166. doi: 10.1016/j.amepre.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abu-Raya B., Giles M.L., Kollmann T.R., Sadarangani M. The effect of timing of tetanus-diphtheria-acellular pertussis vaccine administration in pregnancy on the avidity of pertussis antibodies. Front Immunol. 2019;10:2423. doi: 10.3389/fimmu.2019.02423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naidu M.A., Muljadi R., Davies-Tuck M.L., Wallace E.M., Giles M.L. The optimal gestation for pertussis vaccination during pregnancy: a prospective cohort study. Am J Obstet Gynecol. 2016;215:237.e1–237.e6. doi: 10.1016/j.ajog.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Eberhardt C.S., Blanchard-Rohner G., Lemaître B., et al. Maternal immunization earlier in pregnancy maximizes antibody transfer and expected infant seropositivity against pertussis. Clin Infect Dis. 2016;62:829–836. doi: 10.1093/cid/ciw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petousis-Harris H., Walls T., Watson D., Paynter J., Graham P., Turner N. Safety of Tdap vaccine in pregnant women: an observational study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halperin S.A., Langley J.M., Ye L., et al. A randomized controlled trial of the safety and immunogenicity of tetanus, diphtheria, and acellular pertussis vaccine immunization during pregnancy and subsequent infant immune response. Clin Infect Dis. 2018;67:1063–1071. doi: 10.1093/cid/ciy244. [DOI] [PubMed] [Google Scholar]
- 57.Abu Raya B., Srugo I., Kessel A., et al. The induction of breast milk pertussis specific antibodies following gestational tetanus-diphtheria-acellular pertussis vaccination. Vaccine. 2014;32:5632–5637. doi: 10.1016/j.vaccine.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Perrett K.P., Halperin S.A., Nolan T., et al. Impact of tetanus-diphtheria-acellular pertussis immunization during pregnancy on subsequent infant immunization seroresponses: follow-up from a large randomized placebo-controlled trial. Vaccine. 2020;38:2105–2114. doi: 10.1016/j.vaccine.2019.10.104. [DOI] [PubMed] [Google Scholar]
- 59.Ladhani S.N., Andrews N.J., Southern J., et al. Antibody responses after primary immunization in infants born to women receiving a Pertussis-containing vaccine during pregnancy: single arm observational study with a historical comparator. Clin Infect Dis. 2015;61:1637–1644. doi: 10.1093/cid/civ695. [DOI] [PubMed] [Google Scholar]
- 60.Hardy-Fairbanks A.J., Pan S.J., Decker M.D., et al. Immune responses in infants whose mothers received tdap vaccine during pregnancy. Pediatr Infect Dis J. 2013;32:1257–1260. doi: 10.1097/INF.0b013e3182a09b6a. [DOI] [PubMed] [Google Scholar]
- 61.Maertens K., Caboré R.N., Huygen K., Hens N., Van Damme P., Leuridan E. Pertussis vaccination during pregnancy in Belgium: results of a prospective controlled cohort study. Vaccine. 2016;34:142–150. doi: 10.1016/j.vaccine.2015.10.100. [DOI] [PubMed] [Google Scholar]
- 62.Maertens K., Hoang T.T.H., Nguyen T.D., et al. The effect of maternal pertussis immunization on infant vaccine responses to a booster pertussis-containing vaccine in Vietnam. Clin Infect Dis. 2016;63:S197–S204. doi: 10.1093/cid/ciw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention COVID-19 vaccines while pregnant or breastfeeding. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html Available at:
- 64.World Health Organization The Pfizer BioNTech (BNT162b2) COVID-19 vaccine: what you need to know. 2021. https://www.who.int/news-room/feature-stories/detail/who-can-take-the-pfizer-biontech-covid-19--vaccine Available at:
- 65.World Health Organization The Moderna COVID-19 (mRNA-1273) vaccine: what you need to know. 2021. https://www.who.int/news-room/feature-stories/detail/the-moderna-covid-19-mrna-1273-vaccine-what-you-need-to-know Available at:
- 66.Allotey J., Stallings E., Bonet M., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zambrano L.D., Ellington S., Strid P., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Engjom H., Aabakke A.J.M., Klungsøyr K., et al. COVID-19 in pregnancy-characteristics and outcomes of pregnant women admitted to hospital because of SARS-CoV-2 infection in the Nordic countries. Acta Obstet Gynecol Scand. 2021;100:1611–1619. doi: 10.1111/aogs.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elsaddig M., Khalil A. Effects of the COVID pandemic on pregnancy outcomes. Best Pract Res Clin Obstet Gynaecol. 2021;73:125–136. doi: 10.1016/j.bpobgyn.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vousden N., Bunch K., Morris E., et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: a national cohort study using the UK Obstetric Surveillance System (UKOSS) PLoS One. 2021;16 doi: 10.1371/journal.pone.0251123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mullins E., Hudak M.L., Banerjee J., et al. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecol. 2021;57:573–581. doi: 10.1002/uog.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gurol-Urganci I., Jardine J.E., Carroll F., et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021;225:522.e1–522.e11. doi: 10.1016/j.ajog.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.US Food and Drug Administration FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine. 2020. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 Available at:
- 74.US Food and Drug Administration FDA approves first COVID-19 vaccine. 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine Available at:
- 75.US Food and Drug Administration Moderna COVID-19 vaccine. 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine Available at:
- 76.US Food and Drug Administration Janssen COVID-19 vaccine. 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine Available at:
- 77.Royal College of Obstetricians and Gynaecologists Updated advice on COVID-19 vaccination in pregnancy and women who are breastfeeding. 2020. https://www.rcog.org.uk/en/news/updated-advice-on-covid-19-vaccination-in-pregnancy-and-women-who-are-breastfeeding/ Available at:
- 78.Centers for Disease Control and Prevention V-safe COVID-19 Vaccine Pregnancy Registry. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html Available at:
- 79.Poliquin V., Castillo E., Boucoiran I., et al. Statement on COVID-19 vaccination in pregnancy. 2020. https://sogc.org/common/Uploaded files/Latest News/SOGC_Statement_COVID-19_Vaccination_in_Pregnancy.pdf Available at:
- 80.Collier A.Y., McMahan K., Yu J., et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gray K.J., Bordt E.A., Atyeo C., et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225:303.e1–303.e17. doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perl S.H., Uzan-Yulzari A., Klainer H., et al. SARS-CoV-2–Specific antibodies in breast milk after COVID-19 vaccination of breastfeeding women. JAMA. 2021;325:2013–2014. doi: 10.1001/jama.2021.5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beharier O., Plitman Mayo R., Raz T., et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131 doi: 10.1172/JCI150319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pfizer Pfizer and BioNTech commence global clinical trial to evaluate COVID-19 vaccine in pregnant women. 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-commence-global-clinical-trial-evaluate Available at:
- 85.Sangkomkamhang U.S., Lumbiganon P., Laopaiboon M. Hepatitis B vaccination during pregnancy for preventing infant infection. Cochrane Database Syst Rev. 2014;2014:CD007879. doi: 10.1002/14651858.CD007879.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moro P.L., Zheteyeva Y., Barash F., Lewis P., Cano M. Assessing the safety of hepatitis B vaccination during pregnancy in the Vaccine Adverse Event Reporting System (VAERS), 1990-2016. Vaccine. 2018;36:50–54. doi: 10.1016/j.vaccine.2017.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Andrade Carvalho A., Giampaglia C.M., Kimura H., et al. Maternal and infant antibody response to meningococcal vaccination in pregnancy. Lancet. 1977;2:809–811. doi: 10.1016/s0140-6736(77)90736-x. [DOI] [PubMed] [Google Scholar]
- 88.Shahid N.S., Steinhoff M.C., Roy E., Begum T., Thompson C.M., Siber G.R. Placental and breast transfer of antibodies after maternal immunization with polysaccharide meningococcal vaccine: a randomized, controlled evaluation. Vaccine. 2002;20:2404–2409. doi: 10.1016/s0264-410x(02)00061-0. [DOI] [PubMed] [Google Scholar]
- 89.O’Dempsey T.J., McArdle T., Ceesay S.J., et al. Meningococcal antibody titres in infants of women immunised with meningococcal polysaccharide vaccine during pregnancy. Arch Dis Child Fetal Neonatal Ed. 1996;74:F43–F46. doi: 10.1136/fn.74.1.f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCormick J.B., Gusmão H.H., Nakamura S., et al. Antibody response to serogroup A and C meningococcal polysaccharide vaccines in infants born of mothers vaccinated during pregnancy. J Clin Invest. 1980;65:1141–1144. doi: 10.1172/JCI109768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Letson G.W., Little J.R., Ottman J., Miller G.L. Meningococcal vaccine in pregnancy: an assessment of infant risk. Pediatr Infect Dis J. 1998;17:261–263. doi: 10.1097/00006454-199803000-00023. [DOI] [PubMed] [Google Scholar]
- 92.Myers T.R., McNeil M.M., Ng C.S., Li R., Lewis P.W., Cano M.V. Adverse events following quadrivalent meningococcal CRM-conjugate vaccine (Menveo®) reported to the Vaccine Adverse Event Reporting System (VAERS), 2010-2015. Vaccine. 2017;35:1758–1763. doi: 10.1016/j.vaccine.2017.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheteyeva Y., Moro P.L., Yue X., Broder K. Safety of meningococcal polysaccharide-protein conjugate vaccine in pregnancy: a review of the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol. 2013;208:478.e1–478.e6. doi: 10.1016/j.ajog.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 94.Wak G., Williams J., Oduro A., Maure C., Zuber P.L.F., Black S. The safety of PsA-TT in pregnancy: an assessment performed Within the Navrongo health and demographic surveillance site in Ghana. Clin Infect Dis. 2015;61(Suppl5):S489–S492. doi: 10.1093/cid/civ625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.National Health Service Whooping cough vaccination in pregnancy. 2019. https://www.nhs.uk/pregnancy/keeping-well/whooping-cough-vaccination/ Available at:
- 96.Ministry of Health Immunisation for pregnant women. 2021. https://www.health.govt.nz/your-health/healthy-living/immunisation/immunisation-pregnant-women Available at:
- 97.Centers for Disease Control and Prevention Contraindications and precautions for polio vaccination. 2018. https://www.cdc.gov/vaccines/vpd/polio/hcp/contraindications-precautions.html Available at:
- 98.World Health Organization Safety of immunization during pregnancy: a review of the evidence. 2014. www.who.int/vaccine_safety/.../safety_pregnancy_nov2014.pdf Available at: Accessed May 4, 2021.
- 99.Conlin A.M., Bukowinski A.T., Gumbs G.R., Department of Defense Birth and Infant Health Registry Team Analysis of pregnancy and infant health outcomes among women in the National Smallpox Vaccine in Pregnancy Registry who received anthrax vaccine adsorbed. Vaccine. 2015;33:4387–4390. doi: 10.1016/j.vaccine.2015.05.054. [DOI] [PubMed] [Google Scholar]
- 100.Conlin A.M.S., Sevick C.J., Gumbs G.R., Khodr Z.G., Bukowinski A.T. Safety of inadvertent anthrax vaccination during pregnancy: an analysis of birth defects in the U.S. military population, 2003-2010. Vaccine. 2017;35:4414–4420. doi: 10.1016/j.vaccine.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 101.Meaney-Delman D., Zotti M.E., Creanga A.A., et al. Special considerations for prophylaxis for and treatment of anthrax in pregnant and postpartum women. Emerg Infect Dis. 2014;20 doi: 10.3201/eid2002.130611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khan A.I., Ali M., Chowdhury F., et al. Safety of the oral cholera vaccine in pregnancy: retrospective findings from a subgroup following mass vaccination campaign in Dhaka, Bangladesh. Vaccine. 2017;35:1538–1543. doi: 10.1016/j.vaccine.2017.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grout L., Martinez-Pino I., Ciglenecki I., et al. Pregnancy outcomes after a mass vaccination campaign with an oral cholera vaccine in Guinea: a retrospective cohort study. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hashim R., Khatib A.M., Enwere G., et al. Safety of the recombinant cholera toxin B subunit, killed whole-cell (rBS-WC) oral cholera vaccine in pregnancy. PLoS Negl Trop Dis. 2012;6:e1743. doi: 10.1371/journal.pntd.0001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ali M., Nelson A., Luquero F.J., et al. Safety of a killed oral cholera vaccine (Shanchol) in pregnant women in Malawi: an observational cohort study. Lancet Infect Dis. 2017;17:538–544. doi: 10.1016/S1473-3099(16)30523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mulholland K., Suara R.O., Siber G., et al. Maternal immunization with Haemophilus influenzae type b polysaccharide-tetanus protein conjugate vaccine in the Gambia. JAMA. 1996;275:1182–1188. [PubMed] [Google Scholar]
- 107.Englund J.A., Glezen W.P., Thompson C., Anwaruddin R., Turner C.S., Siber G.R. Haemophilus influenzae type b-specific antibody in infants after maternal immunization. Pediatr Infect Dis J. 1997;16:1122–1130. doi: 10.1097/00006454-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 108.Salam R.A., Das J.K., Dojo Soeandy C., Lassi Z.S., Bhutta Z.A. Impact of Haemophilus influenzae type B (Hib) and viral influenza vaccinations in pregnancy for improving maternal, neonatal and infant health outcomes. Cochrane Database Syst Rev. 2015;2015:CD009982. doi: 10.1002/14651858.CD009982.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moro P.L., Museru O.I., Niu M., Lewis P., Broder K. Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women. Am J Obstet Gynecol. 2014;210:561.e1–561.e6. doi: 10.1016/j.ajog.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.D’Acremont V., Tremblay S., Genton B. Impact of vaccines given during pregnancy on the offspring of women consulting a travel clinic: a longitudinal study. J Travel Med. 2008;15:77–81. doi: 10.1111/j.1708-8305.2007.00175.x. [DOI] [PubMed] [Google Scholar]
- 111.Sudarshan M.K., Madhusudana S.N., Mahendra B.J. Post-exposure prophylaxis with purified vero cell rabies vaccine during pregnancy--safety and immunogenicity. J Commun Dis. 1999;31:229–236. [PubMed] [Google Scholar]
- 112.Sudarshan M.K., Madhusudana S.N., Mahendra B.J., Ashwathnarayana D.H., Jayakumary M., Gangaboriah Post exposure rabies prophylaxis with Purified Verocell rabies vaccine: a study of immunoresponse in pregnant women and their matched controls. Indian J Public Health. 1999;43:76–78. [PubMed] [Google Scholar]
- 113.Sudarshan M.K., Giri M.S., Mahendra B.J., et al. Assessing the safety of post-exposure rabies immunization in pregnancy. Hum Vaccin. 2007;3:87–89. doi: 10.4161/hv.3.3.4010. [DOI] [PubMed] [Google Scholar]
- 114.Chutivongse S., Wilde H., Benjavongkulchai M., Chomchey P., Punthawong S. Postexposure rabies vaccination during pregnancy: effect on 202 women and their infants. Clin Infect Dis. 1995;20:818–820. doi: 10.1093/clinids/20.4.818. [DOI] [PubMed] [Google Scholar]
- 115.Chutivongse S., Wilde H. Postexposure rabies vaccination during pregnancy: experience with 21 patients. Vaccine. 1989;7:546–548. doi: 10.1016/0264-410x(89)90280-6. [DOI] [PubMed] [Google Scholar]
- 116.Fayaz A., Simani S., Fallahian V., et al. Rabies antibody levels in pregnant women and their newborns after rabies post-exposure prophylaxis. Iran J Reprod Med. 2012;10:161–163. [PMC free article] [PubMed] [Google Scholar]
- 117.Huang G., Liu H., Cao Q., Liu B., Pan H., Fu C. Safety of post-exposure rabies prophylaxis during pregnancy: a follow-up study from Guangzhou, China. Hum Vaccin Immunother. 2013;9:177–183. doi: 10.4161/hv.22377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fescharek R., Quast U., Dechert G. Postexposure rabies vaccination during pregnancy: experience from post-marketing surveillance with 16 patients. Vaccine. 1990;8:409. doi: 10.1016/0264-410x(90)90120-b. [DOI] [PubMed] [Google Scholar]
- 119.Clarke E., Kampmann B., Goldblatt D. Maternal and neonatal pneumococcal vaccination - where are we now? Expert Rev Vaccines. 2016;15:1305–1317. doi: 10.1586/14760584.2016.1167602. [DOI] [PubMed] [Google Scholar]
- 120.Quiambao B.P., Nohynek H., Käyhty H., et al. Maternal immunization with pneumococcal polysaccharide vaccine in the Philippines. Vaccine. 2003;21:3451–3454. doi: 10.1016/s0264-410x(03)00349-9. [DOI] [PubMed] [Google Scholar]
- 121.Quiambao B.P., Nohynek H.M., Käyhty H., et al. Immunogenicity and reactogenicity of 23-valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine. 2007;25:4470–4477. doi: 10.1016/j.vaccine.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 122.Holmlund E., Nohynek H., Quiambao B., Ollgren J., Käyhty H. Mother-infant vaccination with pneumococcal polysaccharide vaccine: persistence of maternal antibodies and responses of infants to vaccination. Vaccine. 2011;29:4565–4575. doi: 10.1016/j.vaccine.2011.04.068. [DOI] [PubMed] [Google Scholar]
- 123.Lehmann D., Pomat W.S., Combs B., Dyke T., Alpers M.P. Maternal immunization with pneumococcal polysaccharide vaccine in the highlands of Papua New Guinea. Vaccine. 2002;20:1837–1845. doi: 10.1016/s0264-410x(02)00040-3. [DOI] [PubMed] [Google Scholar]
- 124.O’Dempsey T.J., McArdle T., Ceesay S.J., et al. Immunization with a pneumococcal capsular polysaccharide vaccine during pregnancy. Vaccine. 1996;14:963–970. doi: 10.1016/0264-410x(96)00009-6. [DOI] [PubMed] [Google Scholar]
- 125.Shahid N.S., Steinhoff M.C., Hoque S.S., Begum T., Thompson C., Siber G.R. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet. 1995;346:1252–1257. doi: 10.1016/s0140-6736(95)91861-2. [DOI] [PubMed] [Google Scholar]
- 126.Berezin E.N., Lopes C.C., Cardoso M.R.A. Maternal immunization with pneumococcal polysaccharide vaccine: persistence of maternal antibodies in infants. J Trop Pediatr. 2017;63:118–123. doi: 10.1093/tropej/fmw060. [DOI] [PubMed] [Google Scholar]
- 127.Munoz F.M., Englund J.A., Cheesman C.C., et al. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine. 2001;20:826–837. doi: 10.1016/s0264-410x(01)00397-8. [DOI] [PubMed] [Google Scholar]
- 128.Lopes C.C., Berezin E.N., Scheffer D., et al. Pneumococcal nasopharyngeal carriage in infants of mothers immunized with 23V non-conjugate pneumococcal polysaccharide vaccine. J Trop Pediatr. 2012;58:348–352. doi: 10.1093/tropej/fmr107. [DOI] [PubMed] [Google Scholar]
- 129.Lopes C.R., Berezin E.N., Ching T.H., Canuto Jde S., Costa V.O., Klering E.M. Ineffectiveness for infants of immunization of mothers with pneumococcal capsular polysaccharide vaccine during pregnancy. Braz J Infect Dis. 2009;13:104–106. [PubMed] [Google Scholar]
- 130.Daly K.A., Scott Giebink G., Lindgren B.R., et al. Maternal immunization with pneumococcal 9-valent conjugate vaccine and early infant otitis media. Vaccine. 2014;32:6948–6955. doi: 10.1016/j.vaccine.2014.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robert E., Vial T., Schaefer C., Arnon J., Reuvers M. Exposure to yellow fever vaccine in early pregnancy. Vaccine. 1999;17:283–285. doi: 10.1016/s0264-410x(98)00051-6. [DOI] [PubMed] [Google Scholar]
- 132.Nasidi A., Monath T.P., Vandenberg J., et al. Yellow fever vaccination and pregnancy: a four-year prospective study. Trans R Soc Trop Med Hyg. 1993;87:337–339. doi: 10.1016/0035-9203(93)90156-k. [DOI] [PubMed] [Google Scholar]
- 133.Suzano C.E.S., Amaral E., Sato H.K., Papaiordanou P.M. Campinas Group on Yellow Fever Immunization during Pregnancy. The effects of yellow fever immunization (17DD) inadvertently used in early pregnancy during a mass campaign in Brazil. Vaccine. 2006;24:1421–1426. doi: 10.1016/j.vaccine.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 134.Tsai T.F., Paul R., Lynberg M.C., Letson G.W. Congenital yellow fever virus infection after immunization in pregnancy. J Infect Dis. 1993;168:1520–1523. doi: 10.1093/infdis/168.6.1520. [DOI] [PubMed] [Google Scholar]
- 135.Le Doare K., Kampmann B., Vekemans J., et al. Serocorrelates of protection against infant group B streptococcus disease. Lancet Infect Dis. 2019;19:e162–e171. doi: 10.1016/S1473-3099(18)30659-5. [DOI] [PubMed] [Google Scholar]
- 136.Melin P. Neonatal group B streptococcal disease: from pathogenesis to preventive strategies. Clin Microbiol Infect. 2011;17:1294–1303. doi: 10.1111/j.1469-0691.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- 137.Berner R. Group B streptococcus vaccines: one step further. Lancet Infect Dis. 2021;21:158–160. doi: 10.1016/S1473-3099(20)30451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Madrid L., Seale A.C., Kohli-Lynch M., et al. Infant Group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017;65:S160–S172. doi: 10.1093/cid/cix656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baker C.J., Rench M.A., Edwards M.S., Carpenter R.J., Hays B.M., Kasper D.L. Immunization of pregnant women with a polysaccharide vaccine of Group B streptococcus. N Engl J Med. 1988;319:1180–1185. doi: 10.1056/NEJM198811033191802. [DOI] [PubMed] [Google Scholar]
- 140.Absalon J., Segall N., Block S.L., et al. Safety and immunogenicity of a novel hexavalent group B streptococcus conjugate vaccine in healthy, non-pregnant adults: a phase 1/2, randomised, placebo-controlled, observer-blinded, dose-escalation trial. Lancet Infect Dis. 2021;21:263–274. doi: 10.1016/S1473-3099(20)30478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Carreras-Abad C., Ramkhelawon L., Heath P.T., Le Doare K. A vaccine against group B streptococcus: recent advances. Infect Drug Resist. 2020;13:1263–1272. doi: 10.2147/IDR.S203454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sinha A., Russell L.B., Tomczyk S., et al. Disease burden of Group B streptococcus among infants in sub-Saharan Africa: a systematic literature review and meta-analysis. Pediatr Infect Dis J. 2016;35:933–942. doi: 10.1097/INF.0000000000001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.UNAIDS Seizing the moment: tackling entrenched inequalities to end epidemics | global AIDS update. 2020. https://aids2020.unaids.org/report/ Available at: Accessed June 13, 2021.
- 144.Singh T., Otero C.E., Li K., Valencia S.M., Nelson A.N., Permar S.R. Vaccines for perinatal and congenital infections-how close are we? Front Pediatr. 2020;8:569. doi: 10.3389/fped.2020.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fowler K.B., Stagno S., Pass R.F. Maternal immunity and prevention of congenital cytomegalovirus infection. J Am Med Assoc. 2003;289:1008–1011. doi: 10.1001/jama.289.8.1008. [DOI] [PubMed] [Google Scholar]
- 146.Plotkin S., Orenstein W., Offit P., Edwards K.M. 7th ed. Elsevier; 2017. Plotkin’s vaccines. [Google Scholar]
- 147.Sabbaj S., Ghosh M.K., Edwards B.H., et al. Breast milk-derived antigen-specific CD8+ T cells: an extralymphoid effector memory cell population in humans. J Immunol. 2005;174:2951–2956. doi: 10.4049/jimmunol.174.5.2951. [DOI] [PubMed] [Google Scholar]
- 148.Cabinian A., Sinsimer D., Tang M., et al. Transfer of maternal immune cells by breastfeeding: maternal cytotoxic T lymphocytes present in breast milk localize in the Peyer’s patches of the nursed infant. PLoS One. 2016;11 doi: 10.1371/journal.pone.0156762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Myles I.A., Datta S.K. Frontline Science: breast milk confers passive cellular immunity via CD8-dependent mechanisms. J Leukoc Biol. 2021;109:709–715. doi: 10.1002/JLB.3HI0820-406RR. [DOI] [PubMed] [Google Scholar]
- 150.Plotkin S. The history of vaccination against cytomegalovirus. Med Microbiol Immunol. 2015;204:247–254. doi: 10.1007/s00430-015-0388-z. [DOI] [PubMed] [Google Scholar]
- 151.Plotkin S.A., Boppana S.B. Vaccination against the human cytomegalovirus. Vaccine. 2019;37:7437–7442. doi: 10.1016/j.vaccine.2018.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jenks J.A., Nelson C.S., Roark H.K., et al. Antibody binding to native cytomegalovirus glycoprotein B predicts efficacy of the gB/MF59 vaccine in humans. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abb3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Plotkin S.A., Wang D., Oualim A., et al. The status of vaccine development against the human cytomegalovirus. J Infect Dis. 2020;221:S113–S122. doi: 10.1093/infdis/jiz447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shi T., McAllister D.A., O’Brien K.L., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hall C.B., Weinberg G.A., Blumkin A.K., et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–e348. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 156.Aranda S.S., Polack F.P. Prevention of pediatric respiratory syncytial virus lower respiratory tract illness: perspectives for the next decade. Front Immunol. 2019;10:1006. doi: 10.3389/fimmu.2019.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 158.Chu H.Y., Englund J.A. Maternal immunization. Clin Infect Dis. 2014;59:560–568. doi: 10.1093/cid/ciu327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Madhi S.A., Polack F.P., Piedra P.A., et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426–439. doi: 10.1056/NEJMoa1908380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eichinger K.M., Kosanovich J.L., Lipp M., Empey K.M., Petrovsky N. Strategies for active and passive pediatric RSV immunization. Ther Adv Vaccines Immunother. 2021;9 doi: 10.1177/2515135520981516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Capella C., Chaiwatpongsakorn S., Gorrell E., et al. Prefusion F, postfusion F, G antibodies, and disease severity in infants and young children with acute respiratory syncytial virus infection. J Infect Dis. 2017;216:1398–1406. doi: 10.1093/infdis/jix489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Centers for Disease Control and Prevention Fact sheets | infection control & prevention. 2016. https://www.cdc.gov/tb/publications/factsheets/prevention/bcg.htm Available at:
- 163.Bonde U., Joergensen J.S., Lamont R.F., Mogensen O. Is HPV vaccination in pregnancy safe? Hum Vaccin Immunother. 2016;12:1960–1964. doi: 10.1080/21645515.2016.1160178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moro P.L., Zheteyeva Y., Lewis P., et al. Safety of quadrivalent human papillomavirus vaccine (Gardasil) in pregnancy: adverse events among non-manufacturer reports in the Vaccine Adverse Event Reporting System, 2006-2013. Vaccine. 2015;33:519–522. doi: 10.1016/j.vaccine.2014.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wilson E., Goss M.A., Marin M., et al. Varicella vaccine exposure during pregnancy: data from 10 years of the pregnancy registry. J Infect Dis. 2008;197(Suppl2):S178–S184. doi: 10.1086/522136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.