Figure 1: Bumped Kinase Inhibitors (BKIs) exploit the small gatekeeper structural differences to achieve both potency and specificity.
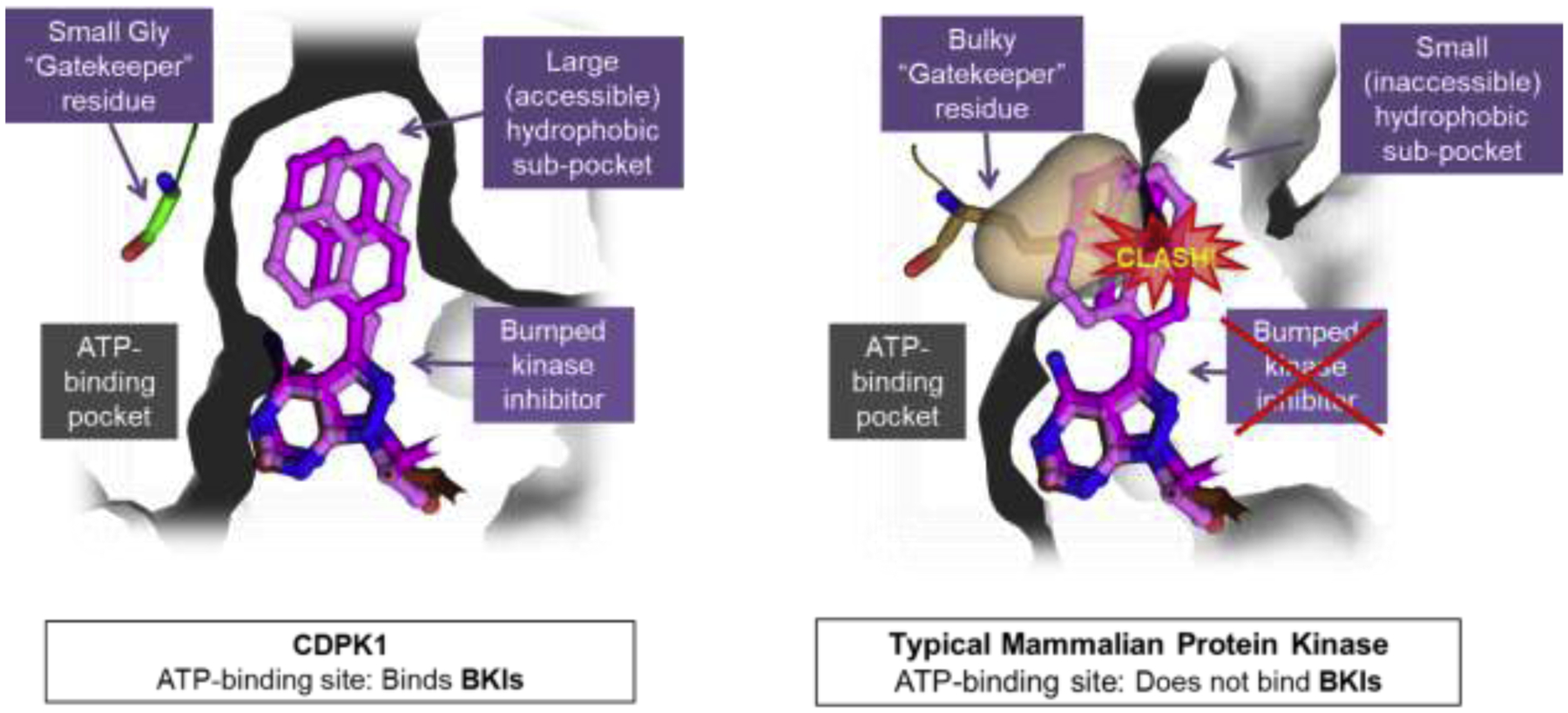
Shown at left is the binding site of T. gondii CDPK1 with a small glycine (Gly) gatekeeper residue, forming a large hydrophobic sub-pocket that allows the BKI to bind at the ATP-binding pocket of CDPK1. On the right is the binding pocket of a typical mammalian kinase with a bulky gatekeeper residue, in this case methionine, demonstrating a clash takes place with the “bump” of BKIs, such that BKIs are excluded from the ATP binding pocket, granting BKIs specificity over almost all mammalian protein kinases.
