Figure 1. Tmem2 variants employed in this study.
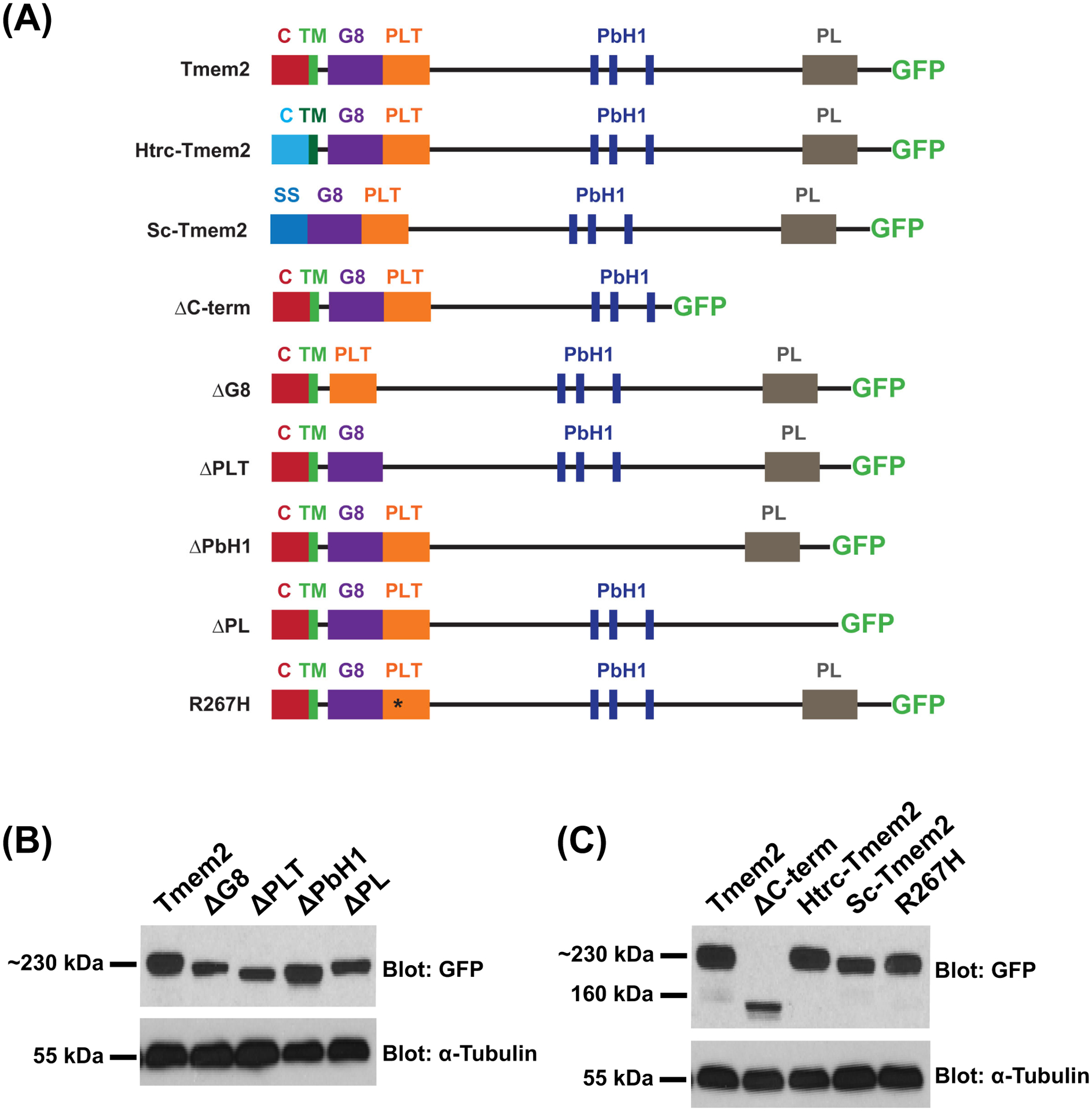
Schematics (A) depict the series of Tmem2-derived variants used in this study. We examined the ability of each to rescue the tmem2 mutant phenotype, relative to the degree of rescue provided by expression of a full-length Tmem2-GFP fusion protein, in which GFP is fused to the C-terminus of Tmem2 (Totong et al., 2011). Tmem2 is predicted to be a type II transmembrane protein and contains a cytoplasmic (C) and transmembrane (TM) domain, a G8 domain, a Pander-like Tmem2 (PLT) domain, three conserved parallel beta-helix repeats (PbH1), and a Pander-like (PL) domain (Smith et al., 2011; Totong et al., 2011). Details of the construction of each variant are provided in the Experimental Procedures section.
Western blots (B,C) compare Tmem2 and Tmem2 variant proteins present in whole cell lysate from transfected HEK293T cells. Amount of protein was normalized across all samples and confirmed by detection of α-Tubulin. Tmem2 and Tmem2 variants were detected via their GFP tag; all Tmem2 variants examined are produced effectively in HEK293T cells and appear to be relatively stable. (B) Tmem2 is approximately 230 kDa, whereas the Tmem2 deletion variants ΔG8, ΔPLT, ΔPbH1, and ΔPL are correspondingly smaller sizes. (C) ΔC-term and Sc-Tmem2 are correspondingly smaller than Tmem2, whereas Htrc-Tmem2 and R267H are approximately 230 kDa. GFP-transfected cell lysate and untransfected cell lysate were also examined as controls (data not shown): the ~27kDa GFP protein was detected in the GFP-transfected sample, whereas no signal was detected in the untransfected sample.
