Figure 9. Hyaluronidase treatment can restrict the distribution of Wnt signaling in Ztmem2 mutants.
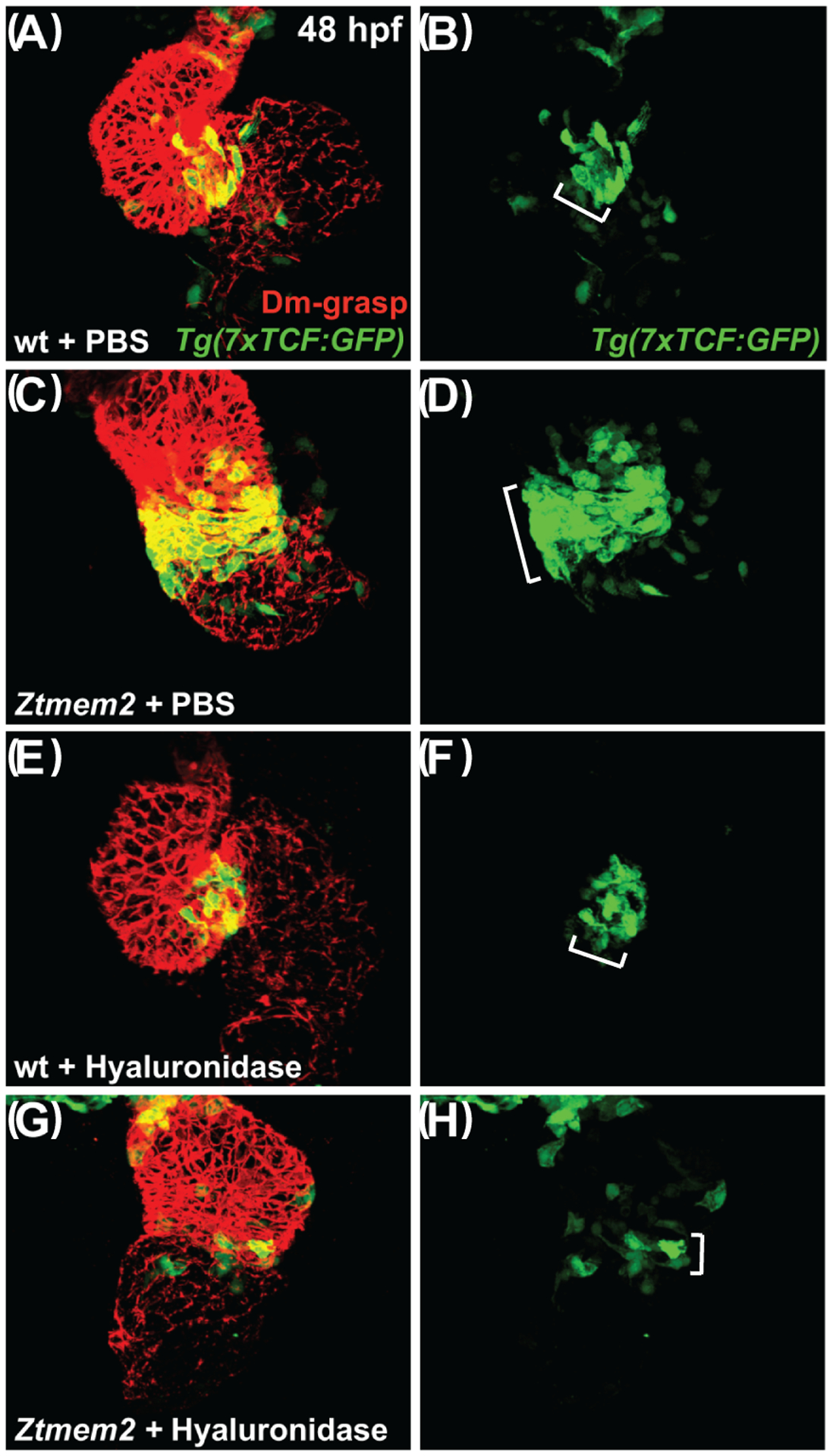
Three-dimensional reconstructions depict immunofluorescent localization of Dm-grasp (red) in the myocardium and GFP (green) driven by the Wnt signaling-responsive reporter transgene Tg(7xTCF-Xla.Siam:GFP) (Moro et al., 2012) in wt (A,B,E,F) and Ztmem2 mutant (C,D,G,H) hearts at 48 hpf. In PBS-injected wt embryos (A,B), Wnt signaling activity was restricted to the AVC (bracket in B). In PBS-injected Ztmem2 mutants (C,D), the distribution of Wnt signaling activity extended beyond its normal boundaries (bracket in D). Although the cytoplasmic localization of the GFP reporter did not facilitate precise quantification, we conservatively estimate that Ztmem2 mutants typically exhibited ~50% more transgene-expressing cardiomyocytes than did their wt siblings. Hyaluronidase treatment restricted the distribution of Wnt signaling activity in the majority of Ztmem2 mutants (G,H; Table 4), but did not affect the Wnt signaling distribution in most wt siblings (E,F; Table 4).
We note that hyaluronidase treatment results in a particularly restricted pattern of Wnt signaling in Ztmem2 mutants (G,H), more restrained than that observed in wt (E,F). While we do not yet understand the basis for this restraint, we speculate that Tmem2 may be required for additional steps of AVC differentiation beyond the restriction of Wnt signaling, consistent with the prior observation that Ztmem2 mutants do not express the AVC differentiation marker spp1 (Smith et al., 2011). In this scenario, while hyaluronidase treatment can rescue the restriction of Wnt reporter activity, it may not be sufficient to rescue additional roles of Tmem2 that are necessary to maintain robust levels of Wnt signaling within the AVC.
