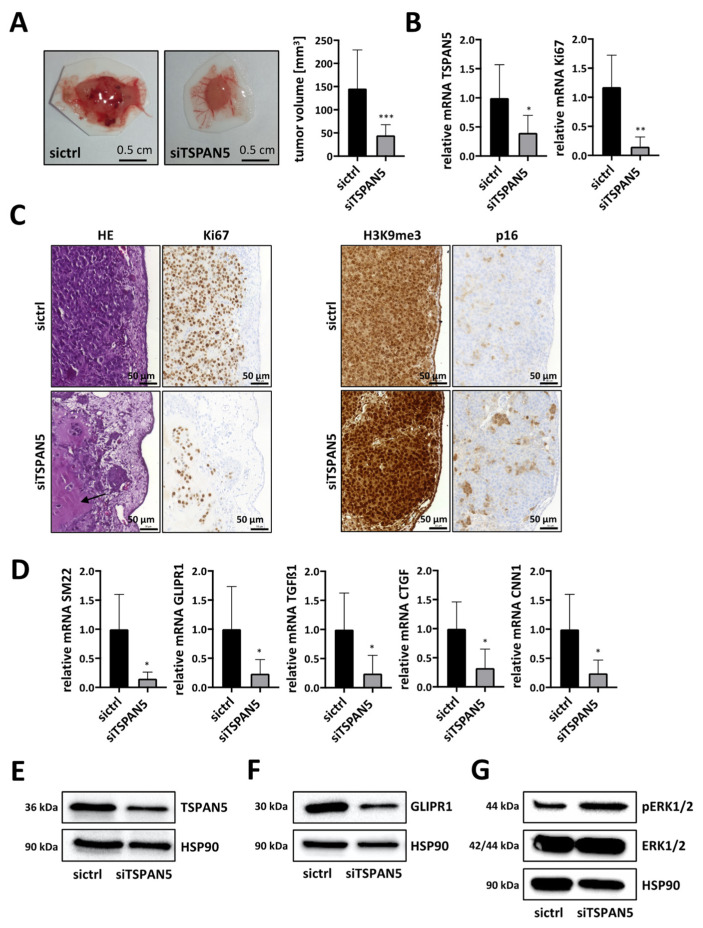
Figure 6.
Anti-tumor effects of TSPAN5 depletion in the CAM assay. (A) HuH7 cells transfected with negative control siRNA (sictrl) and TSPAN5 siRNA (siTSPAN5) in Matrigel® pellets were transferred to the CAM of fertilized chicken eggs. Five days later tumor pellets of HuH7 cells were extracted, ex ovo images of micro-tumors were taken and tumor volume was calculated. Data are presented as means ± SD (n = 17); ***, p < 0.001. (B) Knockdown efficiency of TSPAN5 and Ki67 expression in micro-tumors generated in (A), determined by qRT-PCR using TSPAN5, Ki67 and 18S primers as endogenous housekeeping genes for normalization. Values are means ± SD (n = 6); *, p < 0.05 and **, p < 0.01. (C) Left panel: representative photomicrographs of hematoxylin–eosin (HE)- and Ki67-stained paraffin sections of HuH7 sictrl and siTSPAN5 CAM tumors generated as in (A). Arrowhead: Undigested Matrigel. Scale bar = 50 µm. Right panel: Representative images of H3K9me3- and p16-stained paraffin sections of HepG2 sictrl and siTSPAN5 CAM tumors prepared as in (A). Scale bar = 50 µm. (D) RNA was purified from extracted micro-tumors developed from Matrigel and HuH7 cells treated with scrambled RNA (sictrl) or TSPAN5 siRNA (siTSPAN5) in ovo for 5 days and MRTF target gene expression analyzed by qRT-PCR as described in (B). Values are means ± SD (n = 6); *, p < 0.05. (E–G) Lysates from extracted micro-tumors grown from HuH7 sictrl and HuH7 siTSPAN5 cells in ovo for 5 days as in (A) were immunoblotted with anti-TSPAN5 (E), anti-GLIPR1 (F), anti-ERKpT202/pY204, anti-ERK (G) or anti-HSP90 antibodies as a loading control. The whole Wester Blots are available at Figure S16.