Figure 1. Proposed mechanisms whereby the VWF/ADAMTS13 axis contributes to liver disease.
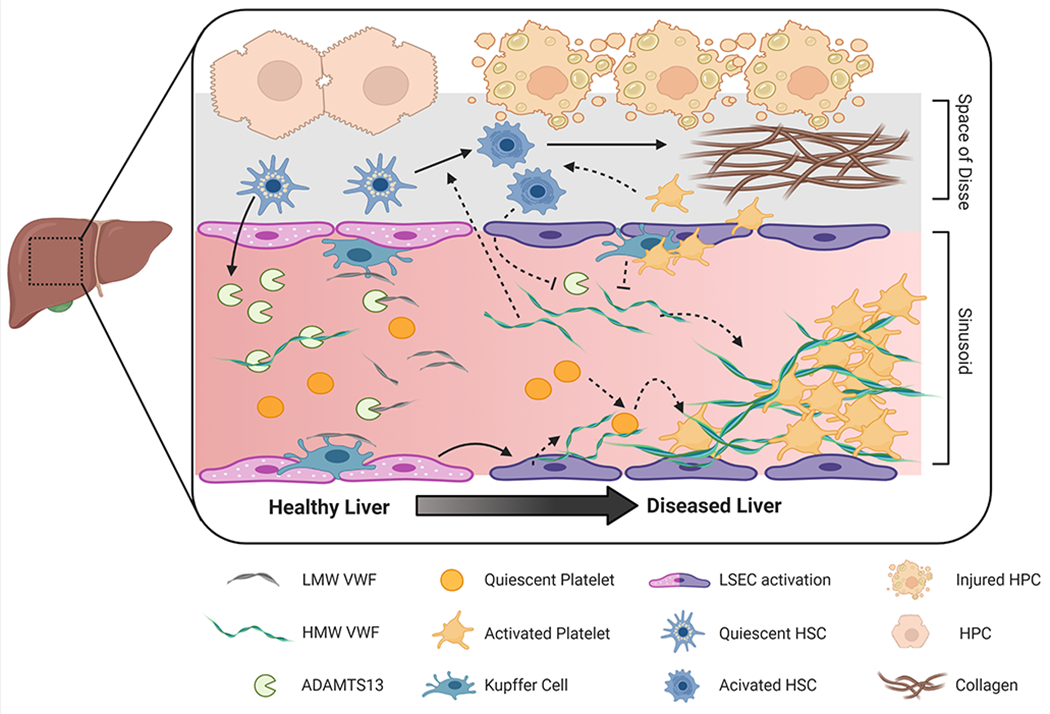
The liver plays a key role in regulating plasma VWF levels. Hepatic stellate cells release ADAMTS13 into the microcirculation where it regulates plasma VWF multimeric size. VWF is also cleared by liver resident macrophages (i.e., Kupffer cells) and hepatocytes. Acute and chronic liver damage impact VWF regulation. For example, loss of endothelial fenestration impairs fluid exchange between hepatocytes and the sinusoidal blood. Plasma ADAMTS13 levels may be altered by disease-dependent changes in expression, secretion, or consumption. Similarly, increased endothelial secretion of VWF combined with impaired clearance by the diseased liver elevates VWF plasma levels. There is also evidence that HMW VWF multimers can be actively consumed and incorporated into platelet-rich microthrombi in the injured liver. Downstream pathologic effects of these microthrombi include disruption of blood flow, exacerbation of tissue injury and delayed liver repair. In addition, VWF and platelets may contribute to hepatic fibrosis by mechanisms dependent or independent of micro-thrombi by amplifying activation of hepatic stellate cells, the primary cell type responsible for exaggerated collagen deposition in chronic liver diseases. LMW, Low-molecular weight; HMW, High molecular weight, LSEC, liver sinusoidal endothelial cell; HSC, hepatic stellate cell; HPC, hepatocyte. Figure created with BioRender.com
