Figure 6.
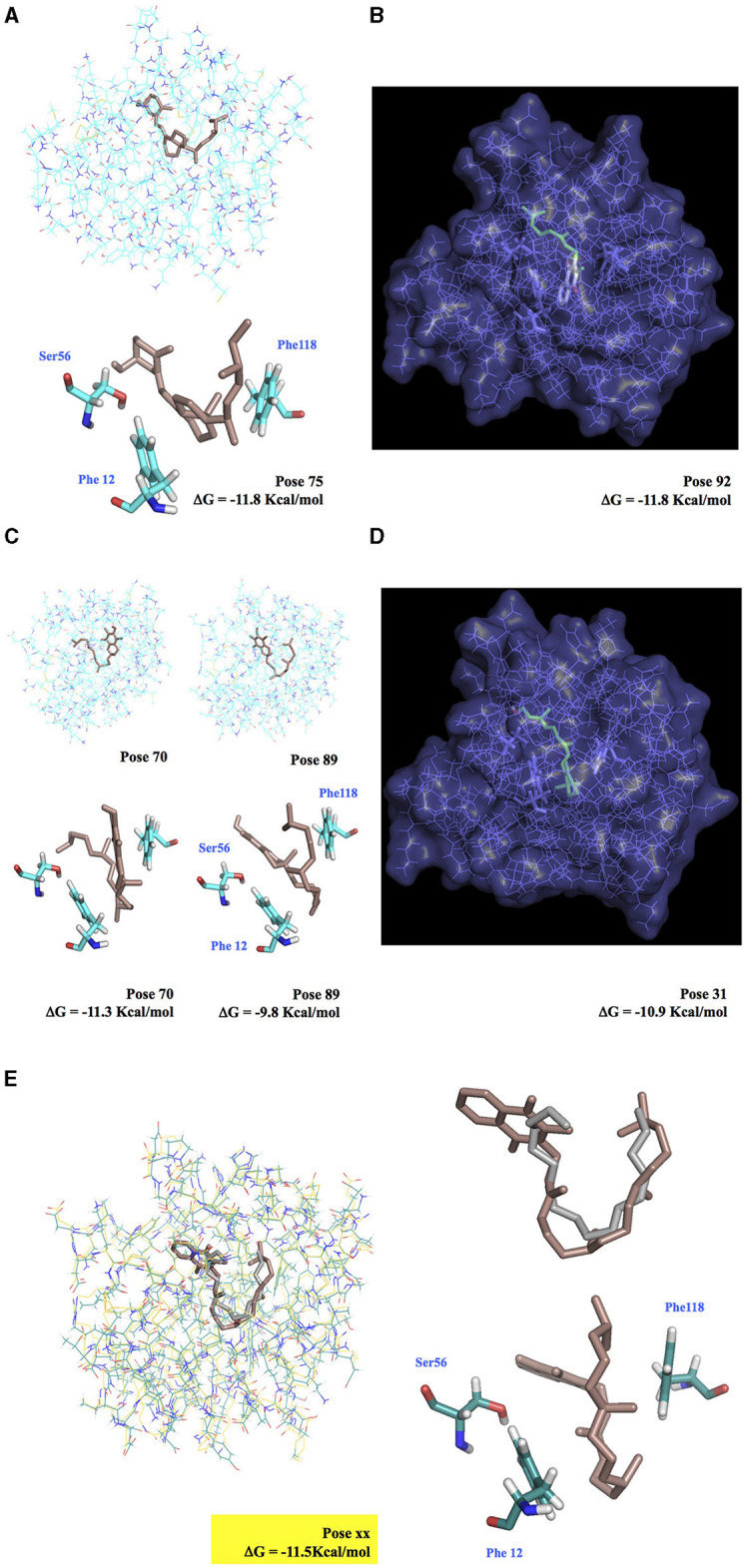
Docking simulation of vitamin molecules integrated into BmorPBP1 binding pocket. (A) Ergocalciferol-BmorPBP1 protein complex. (B) Cutaway view of the interaction of BmorPBP1 with vitamin K2. (C) Vitamin E-BmorPBP1 protein complex in two binding modes. (D) Cutaway view of the interaction of BmorPBP1 with vitamin A. (E) Interaction of BmorPBP1 with vitamin K1 (docking Vina, in brown) and bombykol (X-ray structure, in gray). Vitamin K1 completely overlaps with bombykol. Vitamin K1 attached to Ser56, Phe12, and Phe118 folds in the same position compared with bombykol in the PBP binding site. ΔG shows the relative binding affinity value for vitamin compounds to PBP1. Docking pose shows scored matching or “best-fit” of fragment atoms from vitamin compound and BmorPBP1 in a multiple-grid arrangement.
