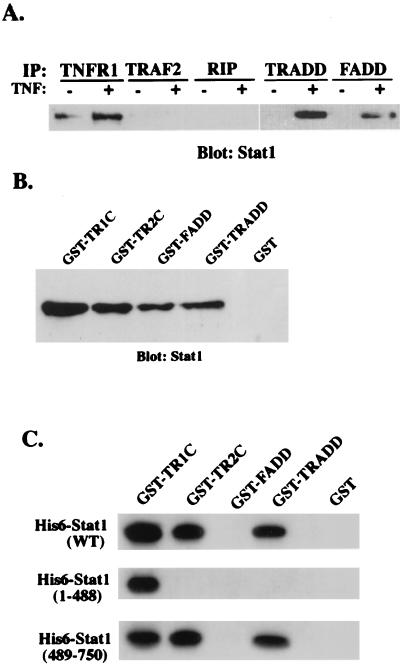
FIG. 2.
Stat1 is a component of the TNFR1-TRADD complex. (A) Whole-cell lysates of HeLa cells (2 × 107) treated or not with TNF-α (10 ng/ml) for 30 min were incubated with protein G-agarose beads conjugated with anti-TNFR1, anti-TRADD, anti-FADD, anti-RIP, or anti-TRAF2 antibody. After a washing, the bead-associated proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Western blotting with anti-Stat1 antibody. IP, immunoprecipitation. (B) Purified forms of GST-TNFR1C, GST-TNFR2C, GST-FADD, GST-TRADD, and GST alone on glutathione-agarose beads were incubated with TNF-α-treated HeLa cell lysates for 2 h at room temperature. After a washing, proteins bound to the beads were subjected to immunoblot analysis with anti-Stat1 antibody. (C) Bacterially expressed His6 epitope-tagged full-length Stat1 (WT) or N-terminally truncated (amino acids 1 to 488) and C-terminally truncated (amino acids 489 to 750) Stat1 proteins were incubated with GST-TNFR1C, GST-TNFR2C, GST-FADD, GST-TRADD, and GST alone, respectively. Precipitated proteins were detected by anti-His6 monoclonal antibody (Amersham Pharmacia).