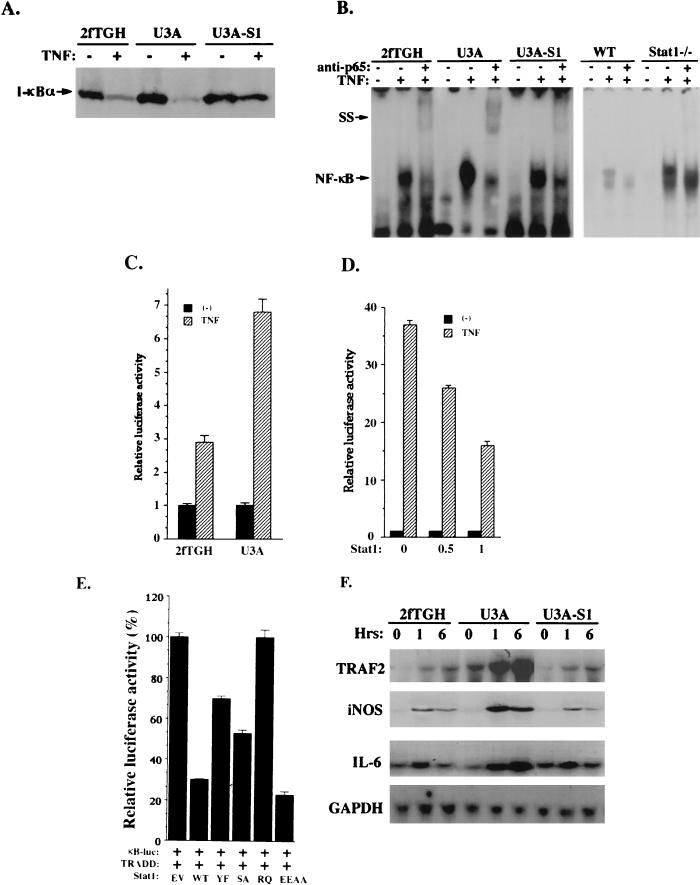
FIG. 5.
Stat1 inhibits NF-κB activation by TNF-α. (A) Protein immunoblot analysis of I-κB-α in whole-cell extracts from 2fTGH, U3A, and U3A-S1 cells treated or not with TNF-α. (B) EMSA were performed as described previously (5) with nuclear extracts prepared from 2fTGH, U3A, and U3A-S1 cells with or without TNF-α treatment. The probe was derived from the NF-κB binding site in the promoter of the I-κB gene. The NF-κB gel shift complex can be supershifted (SS) by the antibody against NF-κB (p65) as indicated. WT, wild type. (C) U3A and 2fTGH cells (3 × 105) were transiently transfected with the 2×κB luciferase reporter construct. Twenty-four hours after transfection, cells were left untreated or treated with TNF-α and luciferase activities were determined. (D) In 293T cells (3 × 105), the 2×κB luciferase reporter was cotransfected with the empty vector (−) or Stat1 expression construct at different concentrations (0.5 and 1 μg). Luciferase activities were analyzed after TNF-α treatment. (E) In 293T cells, the 2×κB luciferase reporter was cotransfected with TRADD and Stat1. Luciferase activities were analyzed after TNF-α treatment. Stat1 proteins tested here include the wild type (WT) and Y701→F mutant (YF), S727→A mutant (SA), R602→Q mutant (RQ), and E495E496→AA mutant (EEAA) forms. (F) Cells were treated with TNF-α for different periods as indicated. Total RNA was isolated and subjected (20 μg) to analysis with probes for the TRAF2, inducible nitric oxide synthase, and interleukin-6 genes. To standardize for the amount of RNA loaded, filters were also probed with the GAPDH gene.