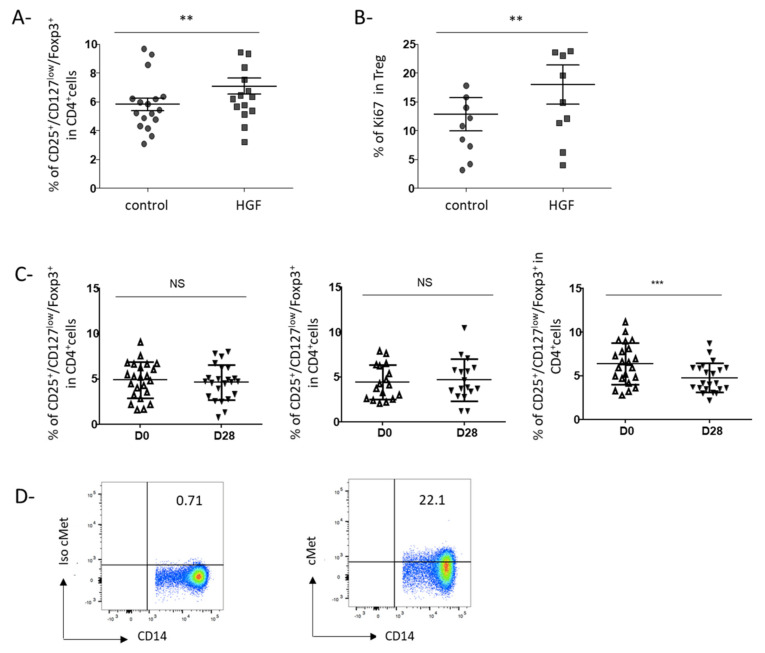
Figure 4.
The proportion of regulatory T cells is enhanced by HGF-DCs in vitro and is decreased by rilotumumab (a monoclonal antibody targeting HGF) in vivo in gastric cancer patients. (A,B) After 6 days of culture, HGF-DCs or control-DCs were then co-cultured with freshly isolated CD4+ T cells from HC. After 7 days of co-culture, cells were collected and their phenotype was assessed by immunostaining and flow cytometry. Treg were defined as CD4+ CD25+ Foxp3+ CD127low cells. Treg proliferation was assessed by Ki-67 intra-cellular expression. (A) The proportion of Treg amongst CD4+ T cells after 7 days of co-culture with control-DCs or HGF-DCs is shown (n = 17). (B) Ki67 was assessed in Treg cells after 7 days of co-culture with control-DCs or HGF-DCs (n = 10). ** indicates a p-value ≤ 0.01 according to the Wilcoxon test. (C,D) The PRODIGE 17-ACCORD 20-MEGA trial is a randomized three-arm phase II study that evaluated the efficacy of 3 treatment regimens in patients with advanced GC: chemotherapy (FOLFOX) alone, an association of FOLFOX + panitumumab (an anti-EGFR monoclonal antibody), or association of FOLFOX + rilotumumab (an anti-HGF monoclonal antibody). PBMCs were collected before (day 1) and after 2 cycles of treatment (day 28). Immunostaining was then performed and cells were analyzed by flow cytometry. Treg was defined as CD4+ CD25+ Foxp3+ CD127low cells, monocytes were defined as CD3−/CD56−/CD19−/CD14+/HLA-DR+ cells as previously described. (C) Proportions of Treg amongst circulating CD4+ cells before and after two cycles of treatment in GC patients treated with FOLFOX (n = 23, left panel), FOLFOX + anti EGFR (n = 17, middle panel) or FOLFOX + anti HGF (n = 21, right panel) are shown. *** indicates a p-value ≤ 0.005 according to the Wilcoxon test. (D) Monocytes from PBMC of 9 GC patients included in the PRODIGE17-ACCORD20-MEGA trial were stained as described in Figure 1 (n = 9). Representative staining out of 9 independent experiments is shown. Results are indicated as percentages of c-Met positive cells.