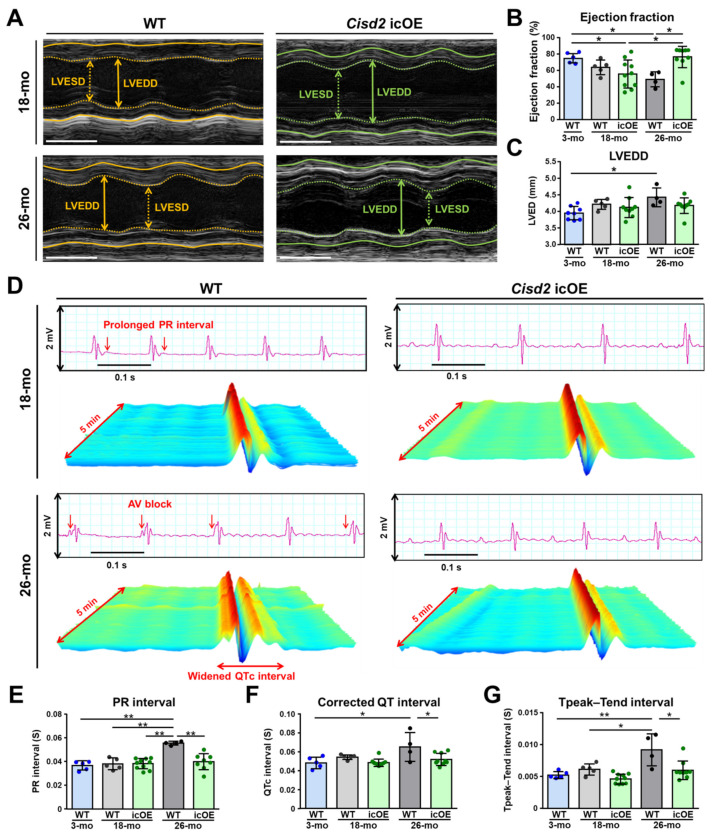
Figure 2.
Cardiac-specific overexpression of Cisd2 at a late-life stage rescues age-related electromechanical dysfunction of the aging heart. (A) Representative echocardiographs obtained from the WT control (R26-Cisd2KI/+) mice and the Cisd2 icOE (R26-Cisd2KI/+; αMHC-MCM) mice (before 18 months of age) and after tamoxifen injection for 8 months (26 months). White bar: 0.1 s. Echocardiographs of the 3-mo WT mice were used as the control. (B,C) Left ventricular ejection fraction (EF) and end-diastolic diameter were measured and quantified by echocardiography. Left ventricular EF decreased in the WT (R26; Cisd2KI/+) mice during aging. However, in the Cisd2 icOE mice, their EF had increased at 26 months of age after induction of Cisd2 overexpression for 8 months compared with that at 18 months. In the WT mice, the LVEDD significantly increased from the age of 18 months to the age of 26 months. However, in the Cisd2 icOE mice, the LVEDD did not increase at 26 months of age compared with 18 months of age. (D) Representative waterfall plots and ECG tracings recorded following anesthesia of the mice. Atrioventricular (AV) block with irregular and progressive prolongation of the PR interval (E), a widened corrected QT (QTc) interval (F), and a prolonged Tpeak–Tend interval (G) were found in the WT mice at 26 months of age. However, cardiac-specific overexpression of Cisd2 appears to have reversed the changes in cardiac function and prevented the heart from suffering from disturbances in electrical conduction. The data are presented as the means ± SD and are analyzed by one-way or two-way ANOVA with Bonferroni multiple comparison tests; * p < 0.05; ** p < 0.005. There were at least five mice in each group. LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter.