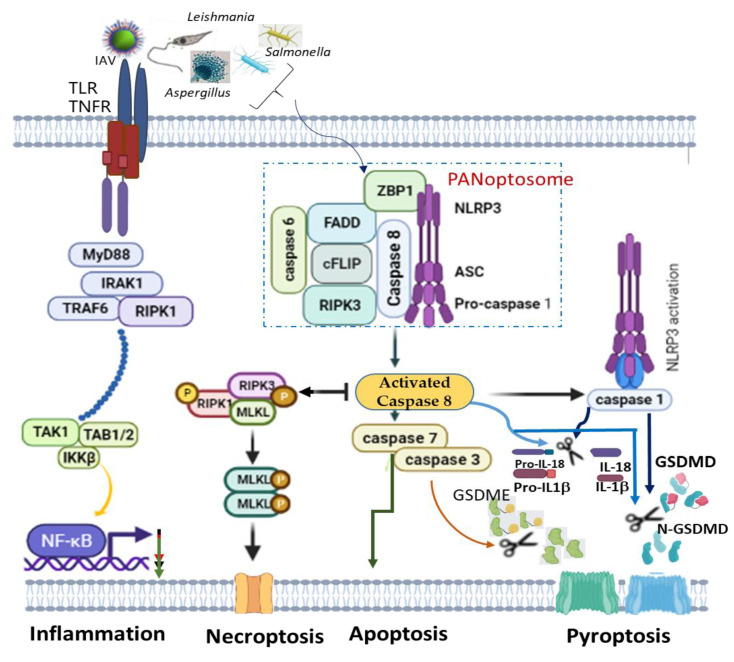
Figure 1.
NLRP3 is an essential component of PANoptosome, and activation of a complex of ZBP1-FADD-RIPK3-caspase 8 drives PANoptosis inflammatory cell death. Activated caspase 8 simultaneously induces apoptosis via downstream activation of caspase 3/7 and directly promotes the assemblage of NLRP3 inflammasome activation complex to initiate pyroptosis via the cleavage of pro-IL1β, pro-IL18 and GSDMD. The reduced activity of the activated caspase 8 drives phosphorylation of MLKL for the induction of necroptosis, while pathogen surface recognition through TLR and TNFR leads to the nuclear binding of NF-κB for inflammation and PANoptosome-independent necroptosis. NLRP3, Nucleotide-binding oligomerization domain; GSDMD, Gasdermin D; GSDME, Gasdermin E; ZBP1, Z-DNA-binding protein 1; RIPK, Receptor-interacting serine/threonine kinase; MLKL, mixed lineage kinase domain-like pseudokinase; FADD, fas-associated death domain; MyD88, myeloid differentiation primary response 88; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; IRAK, interleukin receptor-associated kinase; TAK1, transforming growth factor b-activated kinase 1; TRAF, TNF receptor-associated factor; cFLIP, Cellular caspase-8 (FLICE)-like inhibitory protein.