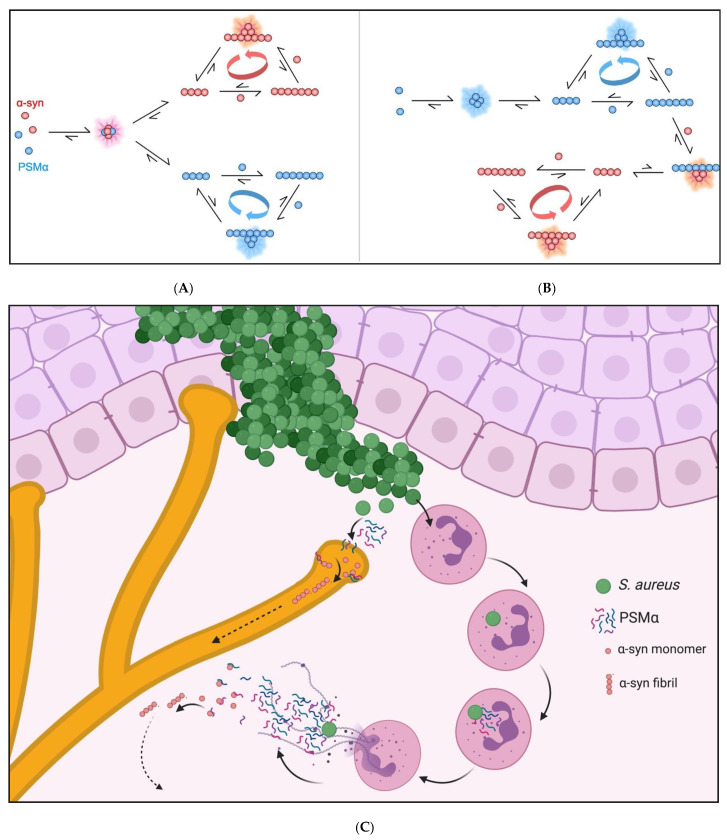
Figure 4.
Schematic illustration of outlining possible modes of PSMα-induced α-syn aggregation. Cartoon illustrating two of many possible modes of cross-catalysis between PSMα (blue) and α-syn (red) involving heterogeneous primary nucleation. The curved arrows indicate the autocatalytic cycles that may result from homogenous secondary nucleation of each peptide on the fibrils of the same peptide. (A) Cross catalysis occurs via heterogeneous primary nucleation in joint oligomers, with no cross-catalysis at fibril level. (B) Separate primary nucleation, significant only for PSMα, and heterogeneous primary nucleation of α-syn on PSMα fibrils. (C) Cartoon illustrating possible routes by which S. aureus and PSMαs could lead to α-syn pathology. Invading S. aureus produces PSMα peptides that are chemoattracting neutrophils. Neutrophils phagocytose S. aureus bacteria, whereby PSMα expression is upregulated leading to phagosome escape and NETosis (24, 25, 27). At high concentrations, the PSMα peptides may interact with extracellular α-syn causing aggregation by enhanced primary nucleation, leading to the formation of fibrils, which can propagate and seed α-syn in other cells (this study). PSMα peptides also form pores in sensory neurons (28, 29). The PSMα peptides may then enter cells and interact with intracellular α-syn.