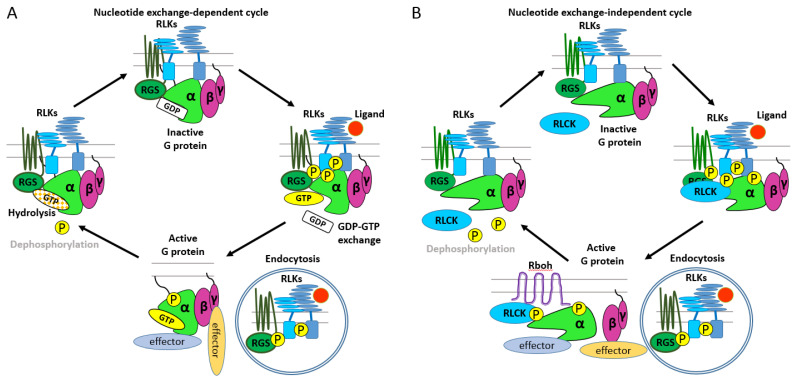
Figure 3.
Model for heterotrimeric G protein signalling in plants. (A) Nucleotide exchange-dependent cycle. The heterotrimer consisting of GDP-bound canonical Gα and the Gβγ dimer is associated with a 7TM-RGS and RLKs at the plasma membrane in its resting state. Upon ligand binding, RLKs phosphorylate Gα and RGS. Ligand binding is followed by receptor and RGS endocytosis and subsequent de-repression of Gα, which releases GDP and binds GTP. GTP-bound Gα does not necessarily separate from Gβγ, and each of the two components modulate downstream signalling cascades. Dephosphorylation (research is urgently needed) and binding to a new RGS leads to GTP hydrolysis terminating signalling and returning the heterotrimer to the inactive state. (B) Nucleotide exchange-independent cycle. The heterotrimer consisting of either canonical Gα or XLG and the Gβγ dimer is associated with a 7TM-RGS (the role of RGS here is yet to be determined), RLKs, and RLCKs at the plasma membrane in its resting state. Upon ligand binding, RLKs phosphorylate Gα/XLG, RGS, and RLCKs. Ligand binding is followed by receptor endocytosis. Here, activation could be caused by de-repression or phosphorylation-mediated activation of Gα/XLG or Gβγ (the activation mechanism is not established). In case of Arabidopsis, XLG2 dissociates from Gβγ upon perception of flg22 [85]. Both Gα/XLG and Gβγ modulate downstream signalling cascades. One example of effector activation is the BIK1/XLG2-mediated activation of RbohD [85]. Hypothetically, dephosphorylation (research is urgently needed) leads to signal termination and reassociation of the heterotrimer into the inactive state.