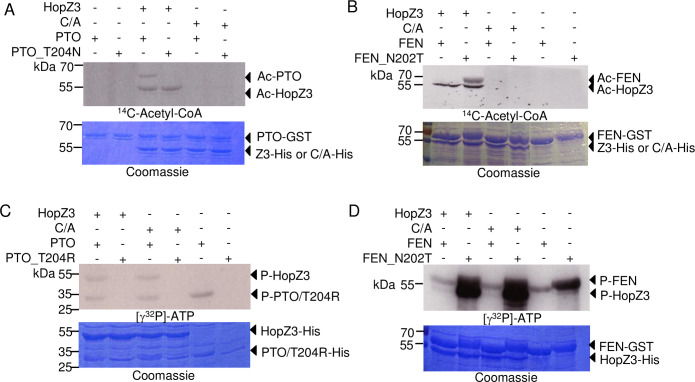
Fig 7. Substitutions in the P+1 activation loop of PTO and FEN affect their acetylation by HopZ3 and their kinase activity.
Purified PTO-GST and FEN variants were incubated with HopZ3-His or HopZ3_C300A (C/A) mutant in the presence of IP6 and 14C-acetyl-CoA (A–B) or γ32P-ATP (C-D). Samples were separated by SDS-PAGE and subjected to autoradiography. (A) PTO but not a T204N P+1 activation loop variant was acetylated by HopZ3. (B) The substitution of Asn202 to Thr in FEN conferred acetylation by HopZ3. (C–D) Kinase activity assay showing PTO and FEN autophosphorylation and transphosphorylation of HopZ3 and HopZ_C300A in vitro. Kinase variants with Thr (wild-type PTO and FEN_N202T) were more active than Arg or Asn variants.