Table 1.
Tyrosine kinase inhibitors, their targets, and application in specific malignancies.
| Generation of TKIs 1 | Name | Chemical Structure | First FDA 2 Approval | Target | Disease | Children | Adults |
|---|---|---|---|---|---|---|---|
| I | Imatinib |
|
2001 | BCR-ABL1 3 | CML 4 | [33,34] | [35,36] |
| Ph+ ALL 5 | [37] | [38] | |||||
| GIST 6 | [39] | [40,41] | |||||
| Brainstem gliomas, intracranial gliomas | [42] | [43] | |||||
| Crizotinib |
|
2011 | ALK 7, ROS1 8, c-MET 9 | ALK-positive lung cancer | No data | [44] | |
| Intrinsic pontine glioma | [45] | No data | |||||
| Solid tumours (hepatocellular carcinoma, adrenal cortical carcinoma, Wilms’ tumour, neuroblastoma ganglioneuroblastoma, rhabdomyosarcoma, synovial sarcoma, epithelial myoepithelial carcinoma, Ewing sarcoma), anaplastic large-cell lymphoma | [46] | [47] | |||||
| Non-small-cell lung carcinoma | No data | [48] | |||||
| Sunitinib |
|
2006 | VEGFR 10, VEGFR2 11, VEGFR3 12, c- KIT 13, FLT3 14, CSF-1R 15, RET 16 |
Renal cell carcinoma | [49] | [50] | |
| GIST | [51] | No data | |||||
| II | Regorafenib |
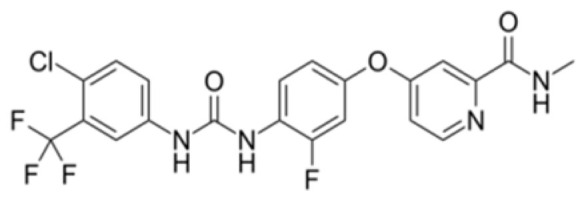
|
2012 | VEGF, EGFR 17 | Liposarcoma | Clinical trial number NCT02085148 | [52] |
| GIST | [53] | ||||||
| Hepatocellular carcinoma | [54] | ||||||
| Dasatinib |
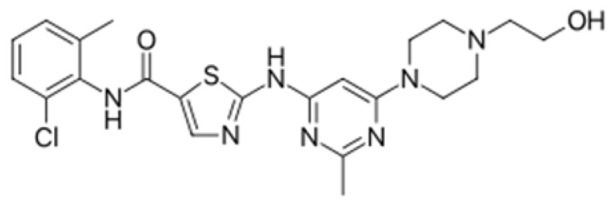
|
2006 | BCR-ABL1, Src 18, c-Kit, ephrin receptors | CML | [55,56] | [57] | |
| Ph+ ALL | [58] | [57] | |||||
| Bosutinib |
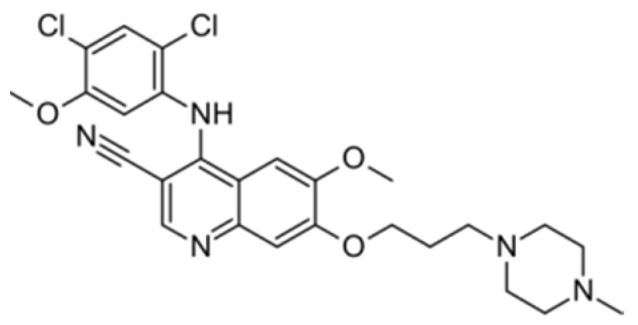
|
2012 | BCR-ABL1, Src | CML | Clinical trial number NCT04258943 | [41,59] | |
| Glioblastoma | No data | [60] | |||||
| Nilotinib |
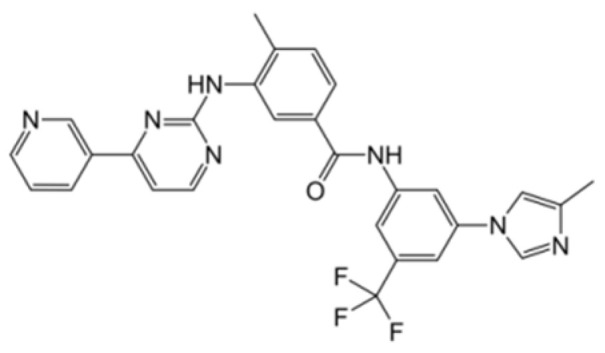
|
2007 | BCR-ABL1 | Ph+ ALL | No data | [58] | |
| CML | [61] | [62] | |||||
| GIST | No data | [63] | |||||
| Glioma | [64] | No data | |||||
| III | Ponatinib |
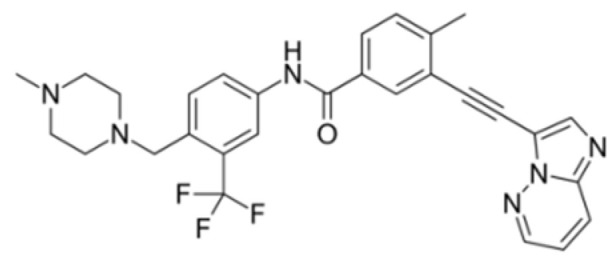
|
2012 | BCR-ABL1 | Glioblastoma | No data | [65] |
| ALL 19 | Clinical trial number NCT04501614 | [66] | |||||
| AML 20 | No data | [67] | |||||
| CML | [68] | [69] | |||||
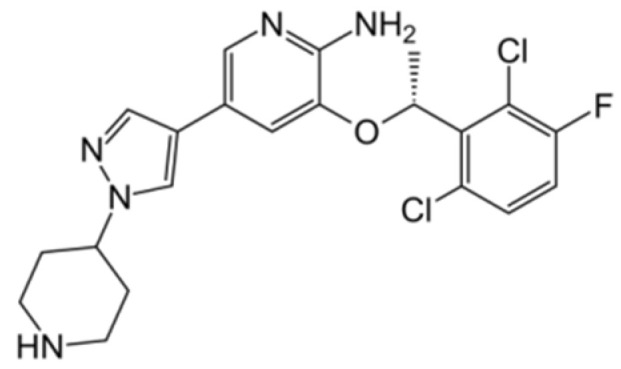
| Osimertinib |
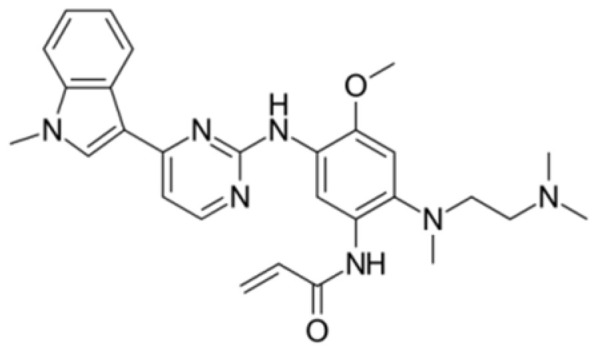
|
2020 | EGFR | Non-small-cell lung carcinoma | No data | [70] | |
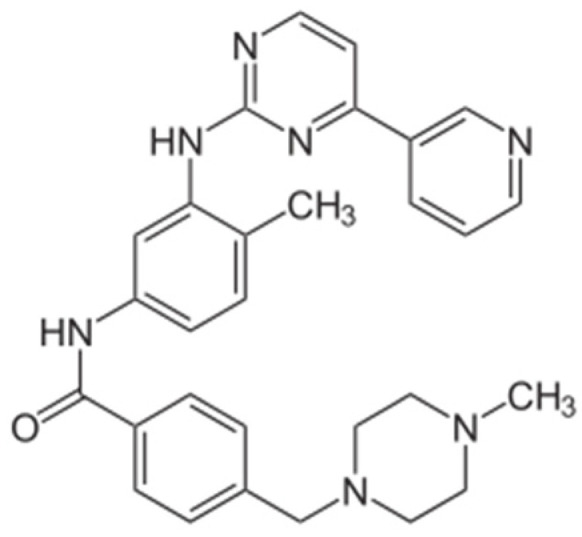
| Olmutinib |
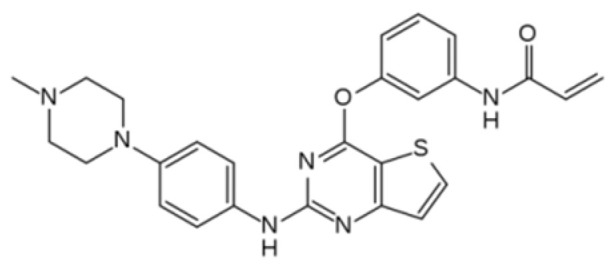
|
No FDA approval, approval in South Korea | No data | [71] | |||
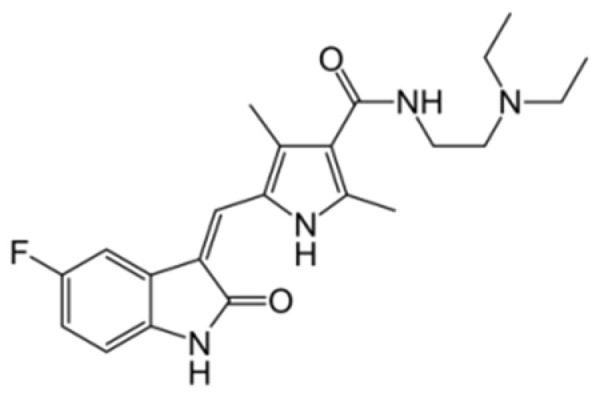
| Nazartinib |
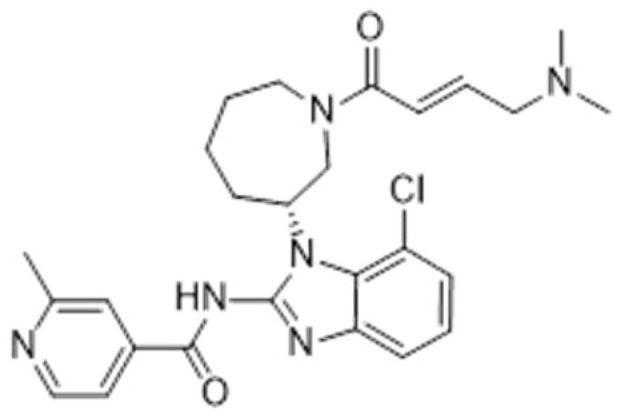
|
No approval, clinical trial number: NCT03040973 | Non-small-cell lung carcinoma, advanced solid tumours | No data | [72] |
1 TKI, tyrosine kinase inhibitor; 2 FDA, Food and Drug Administration; 3 BCR-ABL1, breakpoint cluster region and V-abl Abelson murine leukaemia viral oncogene homolog 1; 4 CML, chronic myeloid leukaemia; 5 Ph+ ALL, Philadelphia-chromosome-positive acute lymphoblastic leukaemia; 6 GIST, gastrointestinal stromal tumour; 7 ALK, anaplastic lymphoma kinase; 8 ROS1, ROS Proto-Oncogene 1, tyrosine kinase receptor; 9 c-MET, hepatocyte growth factor receptor; 10 VEGFR, vascular endothelial growth factor, 11 VEGFR2, vascular endothelial growth factor 2; 12 VEGFR3, vascular endothelial growth factor 3; 13 c-KIT, tyrosine protein kinase KIT; 14 FLT3, cluster of differentiation antigen 135; 15 CSF-1R, colony stimulating factor 1 receptor; 16 RET, proto-oncogene, rearranged during transfection; 17 EGFR, epidermal growth factor receptor; 18 Src, proto-oncogene, non-receptor tyrosine kinase; 19 ALL, acute lymphoblastic leukaemia; 20 AML, acute myeloid leukaemia.
