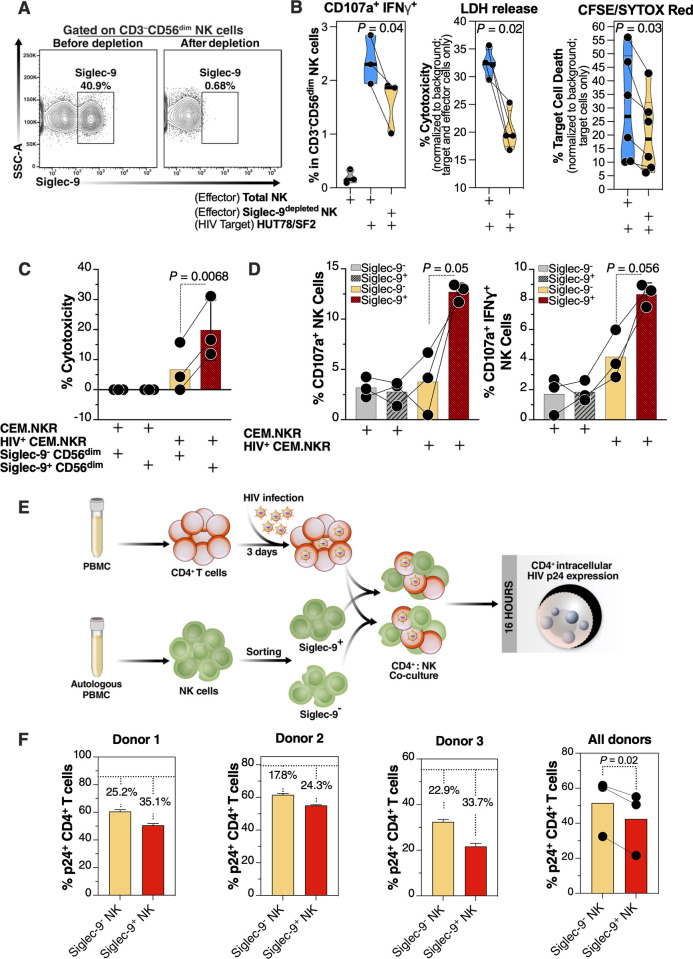
Fig 4. Siglec-9+ CD56dim NK cells exhibit higher cytotoxicity towards HIV+ cells compared to Siglec-9- CD56dim NK cells.
(A) A representative example of depletion of Siglec-9+ NK cells. (B) Siglec-9depleted NK cells exhibit lower cytotoxicity towards HIV-infected HUT78/SF2 targets compared to total NK cells. Cytotoxicity was assessed using NK degranulation, left panel (n = 3 donors; E:T = 4:1), LDH release, middle panel (n = 4 donors; E:T = 10:1), and CFSE/SYTOX Red assay, right panel (n = 6 donors; E:T = 10:1). NK degranulation measured as CD107a+ IFNγ+. Assays from each donor were performed in 2–4 replicates, and the average of these replicates per donor was used for statistical analyses. Statistical analyses were performed using paired t-tests. (C-D) FACS sorted Siglec-9+ CD56dim NK cells exhibit higher cytotoxicity towards HIV+ CEM.NKR targets compared to Siglec-9- CD56dim NK cells. (C) Cytotoxicity was assessed using LDH release assay (n = 3 donors, E:T = 10:1). (D) Analysis of NK degranulation (n = 3 donors; E:T = 4:1) was made on total NK cells gated on Siglec-9+ or Siglec-9- CD56dim NK cell subsets. Siglec-9+ = Siglec-9+ CD56dim NK cells and Siglec-9- = Siglec-9- CD56dim NK cells. Statistical analyses were performed using paired t-tests. (E) A schematic representation of the workflow to evaluate the cytotoxic potential of Siglec-9+ and Siglec-9- CD56dim NK cells against autologous HIV-infected CD4+ T cells. CD4+ T cells were isolated from fresh PBMC and exposed to HIV-1 IIIB for 72 h. On the third day, effector NK cells were isolated from PBMC of the same donor, FACS sorted, and co-cultured with autologous HIV-infected CD4+ T cells for 16 h. Following overnight incubation, the mixtures were stained for live/dead viability, CD3, and intracellular p24. (F) Data from the experimental design shown in (D). Dashed lines denote the percentage of p24+ cells in control HIV-infected CD4+ T cells cultured without effector cells. Percentages are percent reduction from dashed line. Assay from each donor was performed in triplicate (E:T = 10:1; n = 3 donors). Statistical analysis was performed using paired t-test.