Abstract
Background
Health care workers (HCWs) are on the front line for COVID-19. Better knowledge of risk factors for SARS-CoV-2 infection is crucial for their protection. We aimed to identify these risk factors with a focus on care activities.
Methods
We conducted a seroprevalence survey among HCWs in a French referral hospital. Data on COVID-19 exposures, care activities, and protective equipment were collected on a standardized questionnaire. Multivariate logistic regressions were used to assess risk factors for SARS-CoV-2 IgG adjusted on potential confounding.
Findings
Among the 3,234 HCWs enrolled, the prevalence of SARS-CoV-2 IgG was 3.8%. Risk factors included contact with relatives or HCWs with COVID-19 (odds ratio [OR] 2.20 [1.40-3.45] and 2.16 [1.46-3.18], respectively), but not contact with COVID-19 patients. In multivariate analyses, suboptimal use of protective equipment during nasopharyngeal sampling (OR 3.46 [1.15-10.40]), mobilisation of patients in bed (OR 3.30 [1.51-7.25]), clinical examination (OR 2.51 [1.16-5.43]), and eye examination (OR 2.90 [1.01-8.35]) were associated with SARS-CoV-2 infection. Patients washing and dressing and aerosol-generating procedures were additional risk factors, with or without appropriate use of protective equipment (OR 1.37 [1.04-1.81] and 1.74 [1.05-2.88]).
Conclusions
Risk factors for SARS-CoV-2 infection among HCWs are (1) contact with relatives or HCWs with COVID-19, (2) close or prolonged contact with patients, (3) aerosol-generating procedures. Enhanced protective measures during the two latter care-activities may be warranted.
Keywords: Prevention, SARS-CoV-2, Epidemiology, Protective equipment, Nurse specific tasks
Introduction
Since the emergence of SARS-CoV-2 by the end of 2019 in Wuhan, China, health care workers (HCWs) have been on the front lines of the pandemic. Previous publications have reported high percentages of HCWs among patients with coronavirus disease 19 (COVID-19). Initial reports from China found that HCWs represented 3.8% of all cases (1,716 HCWs/44,674).1 In the United States, HCWs represented 16% of the 315,531 cases of COVID-19 among individuals with a known occupational status.2 According to the European CDC, the proportion of HCWs among COVID-19 cases varied from 9% to 26% in several EU countries with available data.3 Finally, a recent meta-analysis reported an overall seroprevalence of SARS-CoV-2 antibodies among HCWs of 8.7% (95% CI [6.7-10.9]).4 In France, 85,456 HCWs were infected by SARS-CoV-2 between March 1, 2020 and July 20, 2021.5
In this context, understanding the main routes of SARS-CoV-2 transmission in HCWs is a major public health question. Several care activities are known or suspected to be associated with increased risk of transmission of most recent coronaviruses (SARS, MERS and SARS-CoV-2), in particular aerosol-generating activities such as intubation, high-flow oxygen, and mechanical ventilation.6 , 7 A study including 2,329 infected HCWs reported that masks were not worn during eye examinations in 47.6% of cases, or during high-risk activities in 19.4% of cases.8 Other circumstances of possible contamination among HCWs were the initial absence of recommendations to wear masks in care settings, or the use of protective equipment only with suspected or confirmed COVID-19 patients.9 , 10 Since these first studies, numerous data have been published. In a recent meta-analysis of 97 studies,11 inappropriate hand-washing, and inadequate or no use of protective equipment were clearly associated with higher risk of SARS-CoV-2 transmission in HCWs. Conversely, care of COVID-19 patients, or work in COVID-19 wards was usually not identified as a risk factor. Current knowledge on the primary routes of SARS-CoV-2 transmission among HCWs remains limited. We aimed to precisely assess main care activities associated with the risk of SARS-CoV-2 infection in HCWs.
Methods
Population and definitions
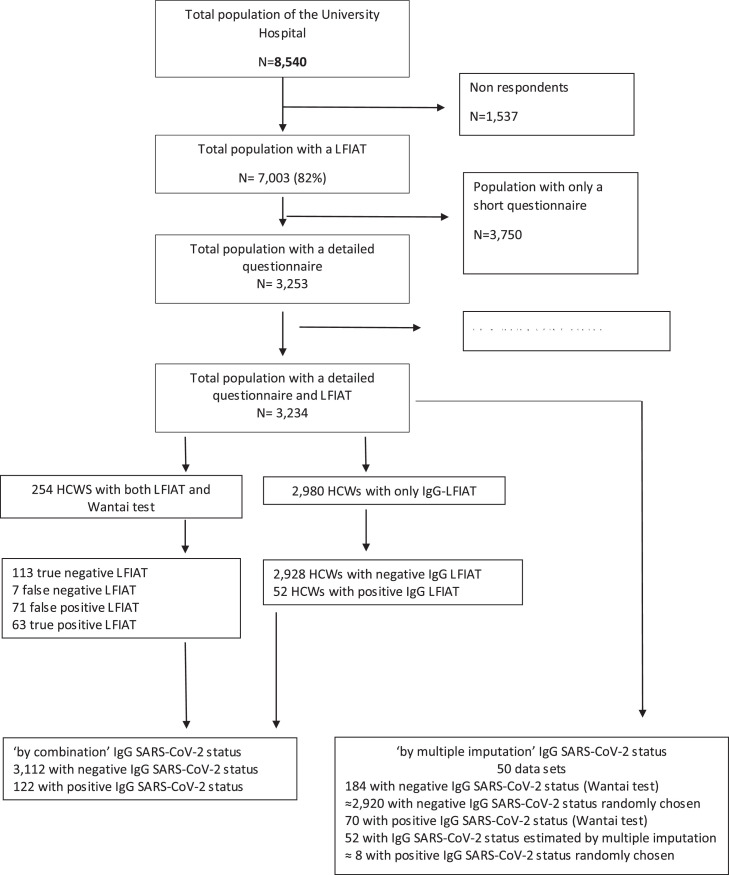
Between May 29 and July 10 2020, we conducted a sero-epidemiological study at the Rennes University Hospital, a 1,500-bed tertiary care centre in western France, which served as a referral center for COVID-19 during the first wave (population catchment area, 1.5 million inhabitants). All HCWs working in the hospital (n = 8,540) were invited to be tested for COVID-19, with a finger-prick rapid test. At inclusion and before the realization of the test, they responded to a short questionnaire with data on sociodemographic characteristic (age, sex, occupation, ward), symptoms, and potential exposure to COVID-19 (n = 7,003, 82% participation rate).
For 1,832 HCWs working in COVID-19 wards, and a random sample of HCWs working in non-COVID-19 wards (n = 1,421), a supplemental questionnaire on occupational exposure was addressed (Fig 1 ), to collect data about the following activities: (1) patients care (consultation, vital sign measurement, insertion of central or peripheral venous catheters, nasogastric tubes, and/or urinary catheters, assistance during delivery, locoregional anaesthesia, clinical examination, nasopharyngeal sampling, oral, eye, and ear, nose, throat (ENT) examination, aerosol-generating procedures, patient mobilisation, bed making, feeding, surgery, distribution of drugs, washing, dressing, mouth care, mobilisation and respiratory physiotherapy, and dental treatment), (2) shared activities with other staff during working hours (transmissions, mealtimes, breaks, meetings and changing-room habits). The use of protective equipment during these activities was also investigated (masks, gloves, lab coats, etc.). Mask use was categorized as appropriate if the HCWs kept the masks, whatever its type, throughout the activity, suboptimal if masks were irregularly used, and absent otherwise.
Fig. 1.
Flow-chart.
Serological status
After completion of the questionnaire, all HCWs underwent a SARS-CoV-2 Lateral Flow ImmunoAssay Test (LFIAT), namely the NG-Test finger-prick test,12 with a reading 20 minutes after the prick by trained nurses or doctors. This test allows the detection of anti-SARS-CoV-2 IgG with a sensitivity of 82.5% and a specificity of 98%.13 If the LFIAT was positive, a venous blood sample (7 mL) was proposed to confirm the serological status (Wantai SARS-CoV-2 Ab ELISA, Beijing, China).
Among the 186 HCWs who responded to the supplemental questionnaire on occupational exposure and who had positive LFIAT, 134 (63%) were additionally tested by a venous blood sample to confirm serological status. In addition, for validation purposes, 120 HCWs with negative LFIAT were randomly selected for a venous blood sample to confirm their negative serology (Fig 1). All data were stored in an on-line database using SPHINX. The study obtained the agreement of the Lyon Institutional Review Board (May 28 2020). All HCWs signed an informed consent form, and the study was recorded on ClinicalTrials.gov (#35RC20_9716).
Statistical analysis
The clinical COVID-19 status was defined as probable if patients presented with fever and dyspnea of acute onset during the first epidemic wave in our area (March-April 2020), and at least one of the following: cough, myalgia, headache or unusual fatigue, or if patients presented with anosmia or ageusia. Patients with other symptoms were defined as possible COVID-19.
The validation study demonstrated that SARS-CoV-2 serological test with LFIAT had a positive predictive value of 49.7% and a negative predictive value of 99.7% for IgG in our population study.13 We thus determined the SARS-CoV-2 serological status (negative / positive) using 2 distinct approaches. First, only positive IgG LFIAT tests were retained to define a positive status. However, as a suitable proportion of HCWs also underwent a Wantai SARS-CoV-2 Ab ELISA (134 HCWs with positive LFIAT and 120 HCWs with negative LFIAT cf. (Fig 1) as a control, we decided to retain this last result when available, irrespective of the LFIAT result. All negative IgG LFIAT tests were considered negative. This definition is thereafter referred to as ‘by combination’, and was used in all descriptive and univariate analyses as well as multivariate analyses. Second, we proceeded as a sensitivity analysis to multiple imputation (n = 50 databases) of the SARS-CoV-2 serological status using a logistic regression model based on the clinical COVID-19 status (defined above) for subjects with a positive IgG LFIAT test, except for subjects with a Wantai SARS-CoV-2 Ab ELISA result. Conversely, negative IgG LFIAT tests were randomly imputed positive at a 0.3% frequency, corresponding to the false negative rate with this test in our validation study. Consequently, serological status, for HCWS who had no Wantai SARS-CoV-2 Ab Elisa test were imputed based on missing at random hypotheses (MAR) and using information on LFIAT, its positive and negative predictive values in our population, and clinical status among positive LFIAT. This definition is thereafter referred to as ‘by multiple imputation’ and only used when multiple approaches were applied.
An analysis of risk factors associated with the serological status was performed using logistic regression models. The descriptive and primary analyses used the ‘by combination’ definition of serological SARS-CoV-2 status, whereas the analyses of overall and occupational risk factors used both definitions. Factorial analysis was first performed for the analysis of activities, as preliminary analyses demonstrated strong correlations among these data (not shown). Six factors were defined corresponding to nurse, auxiliary-nurse, medical, surgical, physiotherapist and ENT activities. Associations between a positive SARS-CoV-2 status and the use of protective equipment were then analyzed by separate backward logistic regression models according to each factor. All models were adjusted for age, sex, and occupation and, depending on analyses, contact with patients, or relatives, diagnosed with COVID-19.
Statistical analyses were performed using the SAS package, v9.4, with FACTOR, LOGISTIC and MIANALYSE procedures. Results are presented as odds ratio (OR) with their 95% confidence intervals (CI). A P value below .05 was considered as significant.
Results
Among the 3,234 HCWs who fulfilled the supplemental questionnaire concerning self-reported occupational exposure and underwent LFIAT (Fig 1), 120 (3.8%) presented IgG SARS-CoV-2 according to the ‘by combination’ definition (Table 1 ). We observed close-to-significant differences (P = .06) among occupations, with cleaners, stretcher-bearers and residents having the highest rate of positive tests. There were no significant differences according to sex, age, smoking status, or comorbidities. The number of symptoms closely correlated with the presence of SARS-CoV-2 IgG, as well as the clinical definition of COVID-19 status (Table 2 ).
Table 1.
Overall characteristics of subjects enrolled in the HB AntiCov study (n = 3,234)
| Characteristics | IgG serological status (by combination) |
P-value 1 | |
|---|---|---|---|
| Negative (n = 3112) | Positive (n = 122) | ||
| Sex (DM = 8) | .28 | ||
| Men | 625 (95.6) | 29 (4.4) | |
| Women | 2,481 (96.5) | 91 (3.5) | |
| Age, years (DM = 6) | .14 | ||
| <30 | 817 (95.1) | 42 (4.9) | |
| 30-39 | 865 (96.8) | 29 (3.2) | |
| 40-49 | 767 (96.2) | 30 (3.8) | |
| 50-+ | 659 (97.2) | 19 (2.8) | |
| Occupation (DM = 10) | .06 | ||
| Administrative staff | 232 (97.5) | 6 (2.5) | |
| Cleaners – Stretcher-bearers | 172 (94.0) | 11 (6.0) | |
| Auxiliary Nurses | 658 (96.8) | 22 (3.2) | |
| Nurses / Midwifes | 979 (96.5) | 35 (3.5) | |
| Students | 142 (97.9) | 3 (2.1) | |
| Residents | 133 (93.0) | 10 (7.0) | |
| Medical staff | 322 (97.3) | 9 (2.7) | |
| HCW with patient contact | 200 (97.1) | 6 (2.9) | |
| HCW without patient contact | 267 (94.0) | 17 (6.0) | |
| Total Medical HCWs | 455 (96.0) | 19 (4.0) | |
| Total Non-Medical HCWs | 1,809 (96.4) | 68 (3.6) | |
| Household (DM = 254) | .01 | ||
| Alone | 403 (93.9) | 26 (6.1) | |
| One child, at least | 108 (95.6) | 5 (4.4) | |
| One adult, at least | 754 (96.1) | 31 (3.9) | |
| One adult and one child, at least | 1,607 (97.2) | 46 (2.8) | |
| Smoking status (DM = 104) | .44 | ||
| No | 2,266 (96.1) | 91 (3.9) | |
| Yes, not every day | 272 (95.4) | 13 (4.6) | |
| Yes, every day | 474 (97.1) | 14 (2.9) | |
| Immunodepression (DM = 0) | .80 | ||
| No | 2,996 (96.2) | 118 (3.8) | |
| Yes | 116 (96.7) | 4 (3.3) | |
Note. Data are presented as numbers (%). Bold values: P < .05.
DM, data missing; HCW, health care workers.
Chi-Square test.
Table 2.
Clinical characteristics of subjects enrolled in the HB AntiCoV study (n = 3,234)
| Characteristics | IgG serological status (by combination) |
P-value1 | |
|---|---|---|---|
| Negative | Positive | ||
| Symptoms | <.0001 | ||
| No symptom | 1,103 (98.4) | 18 (1.6) | |
| 1 | 459 (97.7) | 11 (2.3) | |
| 2-3 | 826 (97.5) | 21 (2.5) | |
| 4-6 | 553 (93.9) | 36 (6.1) | |
| 7+ | 173 (83.6) | 34 (16.4) | |
| Median | 1 | 4 | <.0001 |
| COVID-19 (clinical status) | <.0001 | ||
| No | 1,103 (98.4) | 18 (1.6) | |
| Possible | 1,862 (98.0) | 39 (2.0) | |
| Probable | 149 (70.3) | 63 (29.7) | |
| RT-PCR | <0.0001 | ||
| No test | 1,988 (98.2) | 36 (1.8) | |
| Negative | 1,076 (97.6) | 26 (2.4) | |
| Positive | 50 (46.3) | 58 (53.7) | |
| LFIAT (IgG) (DM = 19) | <.0001 | ||
| Negative | 3,026 (99.8) | 7 (0.2) | |
| Positive | 69 (37.9) | 113 (62.1) | |
Note. Data are presented as numbers (%).
DM, data missing; LFIAT, lateral flow immunoassay test.
Chi-Square test.
Overall, in univariate analyses (Table 3 ), working in a COVID-19 ward (relative to working in a non-COVID-19 ward, 3.8% vs 3.2%, P = .50) or taking care of COVID-19 patients (4.0% vs 3.2%, P = .27), were not associated with a positive status for SARS-CoV-2 IgG. Conversely, contact with a HCW who was diagnosed as COVID-19 was associated with seropositivity for SARS-CoV-2 IgG (4.9% vs 2.4%, P = .0004) as was household contact with someone diagnosed with COVID-19 (6.9% vs 3.2%, P = .0006). Activities associated with increased risk of positive IgG SARS-CoV-2 test in univariate analyses (Table 4 ) were clinical examination (P = .01), and the mobilisation of patients in bed (P = .01). Activities not associated (P > .20) with the risk of positive IgG SARS-CoV-2 test in univariate analyses are listed in Supplementary Table 1.
Table 3.
Risk Factors associated with SARS-CoV-2 IgG serological status obtained by combination in the HB AntiCoV Study (univariate analyses, n = 3,083*)
| Risk factors | IgG serological status (by combination) |
P-value | |
|---|---|---|---|
| Negative | Positive | ||
| Work status during the lockdown | .50 | ||
| Not at work | 143 (94.7) | 8 (5.3) | |
| Worked in non-Covid-19 ward | 1,795 (96.5) | 65 (3.5) | |
| Worked in Covid-19 ward | 1,176 (96.2) | 47 (3.8) | |
| Care of Covid-19 patients (DM = 51) | .27 | ||
| No | 1,114 (96.8) | 37 (3.2) | |
| Yes | 1,806 (96.0) | 75 (4.0) | |
| Contact with a Covid-19 patient family member | .84 | ||
| No | 2,799 (96.4) | 106 (3.6) | |
| Yes | 172 (96.6) | 6 (3.4) | |
| Sampling of SARS-CoV-2 (DM=36) | .11 | ||
| No | 2,079 (96.7) | 70 (3.3) | |
| Yes | 858 (95.6) | 40 (4.4) | |
| Contact with a HCW with Covid-19 (DM = 42) | .0004 | ||
| No | 1,441 (97.6) | 36 (2.4) | |
| Yes | 1,488 (95.1) | 76 (4.9) | |
| Contact with a Covid-19 subject at home (DM = 50) | .0006 | ||
| No | 2,598 (96.8) | 86 (3.2) | |
| Yes | 325 (93.1) | 24 (6.9) | |
Note. Data are presented as numbers (%). Bold values: P < .05.
DM, data missing; HCW, health care workers.
HCWs not at work during lockdown were not considered in this analysis.
Table 4.
Specific tasks associated with SARS-CoV-2 IgG serological status obtained by combination in the HB anti-CoV Study (univariate analyses, n = 3,083*, P < .20)
| IgG serological status (by combination) |
P-value | ||
|---|---|---|---|
| Negative | Positive | ||
| Noncare activities | |||
| Transmissions (DM = 38) | .13 | ||
| No | 379 (98.2) | 7 (1.8) | |
| Yes, suboptimal protective equipment | 1,084 (96.4) | 40 (3.6) | |
| Yes, appropriate protective equipment | 1,431 (96.0) | 59 (4.0) | |
| Meals (DM = 207) | .16 | ||
| No | 381 (96.2) | 15 (3.8) | |
| Yes, suboptimal protective equipment | 955 (97.2) | 27 (2.8) | |
| Yes, appropriate protective equipment | 1,435 (95.8) | 63 (4.2) | |
| Care activities | |||
| Clinical examination (DM = 5) | .01 | ||
| No | 1,709 (96.9) | 56 (3.1) | |
| Yes, suboptimal protective equipment | 160 (92.5) | 13 (7.5) | |
| Yes, appropriate protective equipment | 1097 (96.2) | 43 (3.8) | |
| Nasopharyngeal sampling (DM = 11) | .09 | ||
| No | 2,356 (96.6) | 82 (3.4) | |
| Yes, suboptimal protective equipment | 40 (90.9) | 4 (9.1) | |
| Yes, appropriate protective equipment | 565 (95.8) | 25 (4.2) | |
| Oral and ENT examination (DM = 20) | .18 | ||
| No | 2,397 (96.6) | 85 (3.4) | |
| Yes, suboptimal protective equipment | 49 (92.5) | 4 (7.5) | |
| Yes, appropriate protective equipment | 505 (95.6) | 23 (4.4) | |
| Eye examination (DM = 10) | .06 | ||
| No | 2,761 (96.6) | 98 (3.4) | |
| Yes, suboptimal protective equipment | 49 (92.5) | 4 (7.5) | |
| Yes, appropriate protective equipment | 151 (93.8) | 10 (6.2) | |
| Actions on upper respiratory tract (DM = 13) | .12 | ||
| No | 2,003 (96.7) | 68 (3.3) | |
| Yes, suboptimal protective equipment | 79 (92.9) | 6 (7.1) | |
| Yes, appropriate protective equipment | 876 (95.8) | 38 (4.2) | |
| Patient mobilization (DM = 7) | .01 | ||
| No | 1,108 (96.6) | 39 (3.4) | |
| Yes, suboptimal protective equipment | 220 (92.8) | 17 (7.2) | |
| Yes, appropriate protective equipment | 1,636 (96.7) | 56 (3.3) | |
| Bed making (DM = 7) | .14 | ||
| No | 1,571 (96.4) | 58 (3.6) | |
| Yes, suboptimal protective equipment | 162 (93.6) | 11 (6.4) | |
| Yes, appropriate protective equipment | 1,231 (96.6) | 43 (3.4) | |
| Feeding (DM = 25) | .08 | ||
| No | 2,038 (96.6) | 71 (3.4) | |
| Yes, suboptimal protective equipment | 99 (92.5) | 8 (7.5) | |
| Yes, appropriate protective equipment | 813 (96.6) | 29 (3.4) | |
Note. Data are presented as numbers (%). Bold values: P < .05.
DM, Data missing.
HCWs not at work during lockdown were not considered in this analysis.
Multivariate analyses (Table 5 ), both using the ‘by combination’ or ‘by multiple imputation’ definitions, confirmed the association between SARS-CoV-2 IgG seropositivity and contact with HCWs (OR 1.51, 95% CI [1.18-1.94], by the ‘multiple imputation definition’) or relatives (OR 1.42 [1.08-1.86]), diagnosed with COVID-19. On multivariate analyses adjusted for age, sex, occupation, and contact with a relative or patient with COVID-19, sub-optimal protective equipment during certain tasks was associated with positive test for SARS-CoV-2 IgG (‘by combination definition’): nasopharyngeal samplings (OR 3.46 [1.15-10.40]), mobilization of patients in bed (OR 3.30 [1.51-7.25]), clinical examination (OR 2.51 [1.16-5.43]), and eye examination (OR 2.90 [1.01-8.35]).
Table 5.
Overall risk factors associated with the SARS-CoV-2 IgG serological status obtained by combination (n = 2,866) or multiple imputation (n = 50 data sets) in the HB Anti-CoV Study
| IgG serological status |
||
|---|---|---|
| By combination | By multiple imputation | |
| Variables | OR [CI 95%] | OR [CI 95%] |
| Immunodepression (yes) | 1.32 [0.47-3.74] | - |
| Household (DM = 251) | ns | |
| Alone | 1.00 | |
| One child, at least | 0.54 [0.17-1.65) | - |
| One adult, at least | 0.58 [0.33-1.03] | - |
| One adult and one child, at least | 0.49 [0.28-0.85] | - |
| Care of Covid-19 patient (yes) | 1.30 [0.78-2.17] | - |
| Contact with family of a Covid-19 patient (yes) | 0.90 [0.38-2.10] | - |
| Covid-19 sampling (yes) | 1.00 [0.63-1.60] | - |
| Contact with a Covid-19 health care worker (yes) | 2.08 [1.33-3.24] | 1.51 [1.18-1.94] |
| Contact with a Covid-19 subject at home (yes) | 2.00 [1.23-3.28] | 1.42 [1.08-1.86] |
Logistic regression models, adjusted for age, sex, occupation. Bold values: P < .05.
The same analyses using the ‘by multiple imputation’ definition confirmed these associations only for mobilization of patients in bed, and clinical examination (Table 6 ). Washing and dressing patients were also associated with increased risk of SARS-CoV-2 IgG seropositivity even with self-declared appropriate use of protective equipment, using both the ‘by combination’ and ‘by multiple imputation’ definitions (OR 2.13 [1.05-4.30], and 1.51 [1.06-2.14], respectively). Finally, aerosol-generating procedures, whether with self-declared appropriate, or suboptimal use of protective equipment, were also associated with positive test for SARS-CoV-2 IgG using the ‘by multiple imputation’ method (OR 1.37 [1.04-1.81] and 1.74 [1.05-2.88], respectively).
Table 6.
Specific care tasks associated with the SARS-CoV-2 IgG serological status obtained by combination (n = 2,961) or multiple imputation (n = 50 data sets) in the HB Anti-CoV Study
| IgG serological status |
||
|---|---|---|
| by combination | by multiple imputation | |
| Variables | OR [CI 95%] | OR [ICI 95%] |
| Nurse specific tasks | ||
| Nasopharyngeal sampling | ||
| No | 1.00 | 1.00* |
| Yes, suboptimal protective equipment | 3.46 [1.15-10.40] | 0.88 [0.32-2.43] |
| Yes, appropriate protective equipment | 1.33 [0.79-2.25] | 1.13 [0.84-1.52] |
| Central lines insertion | ||
| No | 1.00 | 1.00 |
| Yes, suboptimal protective equipment | 2.66 [0.57-12.38] | 1.75 [0.82-3.74] |
| Yes, appropriate protective equipment | 0.72 [0.35-1.45] | 0.74 [0.48-1.14] |
| Actions on upper respiratory tract | ||
| No | 1.00* | 1.00 |
| Yes, suboptimal protective equipment | 1.85 [0.66-5.22] | 1.74 [1.05-2.88] |
| Yes, appropriate protective equipment | 1.53 [0.92-2.55] | 1.37 [1.04-1.81] |
| Auxiliary nurse specific tasks | ||
| Washing and dressing patient | ||
| No | 1.00 | 1.00 |
| Yes, suboptimal protective equipment | 0.38 [0.10-1.46] | 0.63 [0.29-1.36] |
| Yes, appropriate protective equipment | 2.13 [1.05-4.30] | 1.51 [1.06-2.14] |
| Patient mobilization | ||
| No | 1.00 | 1.00 |
| Yes, suboptimal protective equipment | 3.30 [1.51-7.25] | 2.04 [1.33-3.11] |
| Yes, appropriate protective equipment | 0.93 [0.49-1.79] | 1.00 [0.70-1.44] |
| Feeding | ||
| No | 1.00 | 1.00* |
| Yes, suboptimal protective equipment | 2.48 [0.92-6.72] | 1.24 [0.65-2.36] |
| Yes, appropriate protective equipment | 1.00 [0.54-1.89] | 0.87 [0.62-1.24] |
| Medical doctors specific tasks | ||
| Clinical examination | ||
| No | 1.00 | 1.00 |
| Yes, suboptimal protective equipment | 2.51 [1.16-5.43] | 1.62 [1.06-2.48] |
| Yes, appropriate protective equipment | 1.50 [0.85-2.64] | 1.23 [0.90-1.68] |
| Eye examination | ||
| No | 1.00 | 1.00* |
| Yes, suboptimal protective equipment | 2.90 [1.01-8.35] | 1.39 [0.66-2.91] |
| Yes, appropriate protective equipment | 1.86 [0.93-3.73] | 1.02 [0.60-1.72] |
Note. Logistic regression, P-value < .20, adjusted for age, sex, occupation, contact with a Covid-19 patient, contact with a Covid-19 subject at home. Bold values: P < .05.
Each group of specific tasks by occupation corresponded to an independent model according to the factorial analysis (see methods).
Variable not included in the final regression logistic model as P > .20.
Discussion
This large seroepidemiological study, which included more than 3,000 HCWs in a French university hospital after the first epidemic wave, highlights several possible risk factors for SARS-CoV-2 transmission. First, during the first epidemic wave, contact with relatives or HCWs diagnosed with COVID-19 were the 2 main risk factors for SARS-CoV-2 infection, while working in COVID-19 wards or contact with COVID-19 patients was not associated with an increased risk. Second, we confirmed that certain tasks performed by HCWs that increase the risk of aerosolization also increase the risk of SARS-CoV-2 transmission, particularly when use of protective equipment was suboptimal, such as interventions on the upper respiratory tract or nasopharyngeal sampling. Third, we found that other tasks of routine daily care, such as patients washing, dressing, mobilisation, and eye or clinical examinations are associated with increased risk of SARS-CoV-2 infection among HCWs.
A lower risk of transmission when working in dedicated COVID-19 wards during the first epidemic wave has already been reported. In a British cross-sectional study of 545 HCWs, working in an intensive care unit with COVID-19 patients was associated with a lower risk of SARS-CoV-2 infection than working in other wards (OR 0.28 [0.09-0.78].14 A large US cross-sectional study found no risk associated with working in COVID-19 wards (OR 1.00 [0.98-1.03]) or intensive care units (OR 0.98 [0.93-1.02]), among 49,329 HCWs tested for SARS-CoV-2 IgG antibodies.15 A study from China reported a higher risk in HCWs working in non-COVID-19 wards (relative to dedicated wards, IRR 3.1 [1.8-5.2]).16 These findings suggest that working in dedicated wards where all patients are suffering from COVID-19 led to more appropriate use of protective equipment.1 , 17 Of note, one study reported an excess risk in frontline HCWs working in dedicated COVID-19 wards (RR 1.65 [1.34-2.02]) among 28,792 subjects.18 However, that study considered both IgG and IgM antibodies obtained by a self-interpreted LFIA test to be positive, which may not be accurate.
In a sero-prevalence study using a different LFIA test among 3,056 HCWs in a Belgian hospital, contact with COVID-19 patients was not associated with higher prevalence of SARS-CoV-2 IgG antibodies (OR 1.08 [0.80-1.45].19 In addition, Moscola et al15 also did not observe a risk associated with direct patient care. Conversely, Lentz et al17 reported increased risk in HCWs with exposure to COVID-19 patients (OR 1.4 [1.0-1.9]), and that risk was associated with routine contact (OR 1.4 [1.04-1.90]), rather than exposure to aerosol-generating procedures (OR 0.9 [0.6-1.2]). Similarly, Shat et al20 reported a higher risk of COVID-19 for HCWs taking care of COVID-19 patients (HR 3.30 [2.13-5.13]) after adjustment for sex, age, ethnicity, socioeconomic status and comorbidity. Iversen et al18 also reported a mild excess risk for HCWs in contact with COVID-19 patients (RR 1.22 95% [1.03-1.45]).
One explanation for such discrepancies may be the appropriate use of protective equipment. Several at-risk exposures have been reported for SARS, MERS, and SARS-CoV-2. In a large literature review, Chou et al21 highlighted that intubation, direct patient care, and contact with bodily secretions increased the risk of coronavirus infections, but data were less robust for other types of exposure such as noninvasive positive-pressure ventilation, nebulizers use, manipulation of oxygen masks, and high-flow oxygen. This review confirmed the protective effect of using a mask, either surgical or N95,1 as previously reported. For coronaviruses, N95 masks are not generally found to be more protective than surgic.al masks for most at-risk exposures, with a few exceptions.22 , 23 However, these studies suffered from methodological flaws.
Our study suggests an increased risk associated with aerosol-generating procedures such as nasopharyngeal sampling, in line with the recommendation to use N95 masks for these procedures. Moreover, actions on the upper respiratory tract were associated with increased risk of SARS-CoV-2 infection, with or without protective equipment (OR 1.37 [1.04-1.81], and 1.74 [1.05-2.88], respectively, using the ‘by multiple imputation’ definition). We also found a higher risk of SARS-CoV-2 IgG positivity associated with two auxiliary-nurses activities, namely patients washing and dressing, and their mobilisation in bed. The masks routinely used while performing these activities are surgical, and our results suggest that this level of protection may not be appropriate. Indeed, these activities require close, and prolonged contact with patients, two documented risk factors for SARS-CoV transmission.22 , 24 , 25
For SARS-CoV-2, to the best of our knowledge, only two studies suggested that duration of care may be a risk factor for infection. Lentz et al17 documented an increased risk associated with care longer than 45 minutes. Grant et al26 found higher prevalence of SARS-CoV-2 antibodies among HCWs with exposure defined as prolonged direct contact with patients. Another explanation may be the generation of a small amount of SARS-CoV-2 aerosol,27 during such care activities, in particular by patients not wearing a mask, responsible for contamination by inhalation, despite the use of a surgical mask. These findings suggest that protective equipment must be reinforced when HCWs are exposed to prolonged and close care of COVID-19 patients. Finally, we also observed higher risk associated with eye and clinical examinations. These results are consistent with those of the literature,7 as SARS-CoV-2 can be detected in tears and conjunctival secretions,28 and eye examination requires close contact. Suboptimal use of protective equipment under these conditions may place HCWS at risk. Our study is, to our knowledge, the first to suggest that clinical examination may be associated with increased risk of SARS-CoV-2 transmission, even with self-declared appropriate use of protective equipment. This may also be possibly explained by close contact and, to a lesser extent, the duration of contact.
We found an increased risk of SARS-CoV-2 infection in HCWs with household relatives diagnosed with COVID-19 (OR 1.42 [1.08-1.86]). Lentz et al found a significant risk of infection associated with exposure outside of work, including living with a household member diagnosed with COVID-19 (OR 3.8 [1.5-9.3]) or who presented COVID symptoms (OR 3.1 [1.5-6.3]). Lai et al. also reported more frequent contact with confirmed cases of COVID-19 among family members than colleagues, albeit the difference was nonsignificant (12.7% vs 10.9%). Treibel et al29 compared the number and incidence of patients positive for SARS-CoV-2 in Greater London, to that of HCWs in their cohort study, and suggested that these data likely reflect general community transmission than in-hospital exposure. Finally, Steensels et al19 found an association between sero-prevalence of SARS-CoV-2 antibodies and contact with suspected COVID-19 households (OR 3.15 [2.33-4.25]).
Our study has limitations. First, data on the use of protective equipment, particularly masks, were only declarative, and some HCWs may have over- or under-reported their use. We tried to limit this effect by attributing the quality of protection independently of the tasks using the same algorithm throughout the database. However, we observed associations for only a few activities, and the observation of a coherent gradient of transmission risk with the quality of protection supports the validity of our findings. Another limitation was the low sero-prevalence, resulting in a low statistical power. Nonetheless, we were able to highlight several activities associated with the risk of SARS-CoV-2 infection, even in multivariable analyses. We also only considered SARS-CoV-2 IgG, as the LFIAT has low performances for the detection of SARS-CoV-2 IgM.12 Hence, we may have underdiagnosed recent SARS-CoV-2 infection. However, as we began our study at the end of May, two months after the peak of the first epidemic wave in France, the effect on our prevalence estimate was probably minimal. Finally, we only analysed the protective effect of masks, without considering gloves, visors, and lab coats. Thus, our results primarily apply to risk of SARS-CoV-2 transmission by inhalation.
Our study also has strengths. The determination of SARS-CoV-2 status was based on a LFIA test that we previously validated.13 Our knowledge of the quality of both the negative and positive predictive values in the study population allowed us to include these data in our models, using multiple imputation after stratification on the LFIA test response. This method, generally used to complete missing data, was a good tool to correct our sero-prevalence results according to the validation study. Moreover, several authors have recommended accounting for such errors.30 , 31 Thus, despite differences between the ‘in combination’ and ‘multiple imputation’ definitions, these approaches provide more confidence in our results. Another strength was the selection of HCWs enrolled in the study. Our sample is representative of HCWs of our hospital as it included all voluntary HCWs within COVID-19 wards and a random selection of those working in non-COVID-19 wards. Moreover, the high rate of participation (>80%) ensure that our sample was representative. A comparison of demographic and occupational characteristics between respondents and non-respondents did not find any difference (data not shown).
Our study allowed to precisely study the role of several care-associated activities, including nursing and auxiliary-nursing care. Our methodology, using factor analyses coupled to multivariate logistic regression also allowed to take into account statistical correlations among the variables. However, residual correlations may explain some of the variation in the observed associations between specific care activities and the presence of SARS-COV-2 antibodies. As already mentioned, to the best of our knowledge, no previous study on this subject have used these statistical approaches.
Conclusions
This study highlights several possible routes of SARS-CoV-2 transmission associated with specific medical, nursing or auxiliary-nursing activities. Although protective equipment was appropriately used by most HCWs, these findings support the possible role of less known situations in the transmission of SARS-CoV-2. In particular, long-duration, non-aerosol generating activities close to patients, such as mobilising them in their beds, washing and dressing, and clinical or eye examinations may be at higher risk than previously thought. Better use of adequate protective equipment during these activities must be encouraged to better protect HCWs. Further studies are required to better understand the routes of SARS-CoV-2 transmission among HCWs.
Acknowledgments
We thank all participants and nurses (Anne-Marie Le Bonniec, Sabine Filande, Veronique Grigoli, and Gaelle Oudard), and residents (Nadia Fatih, Alexandre Bichon, Jean Poinsignon, Annabelle Guilloux, Léah Rakotonirina and Ahmed Aiouaz) of the occupational medicine unit for inclusion of the participants. We thank Pr Boudjema, Pr Malledant, Nicolas Mevel, Sophie Huitorel, Agnes Gazzola, Anne-Sophie Jouault, and Valerie Turmel for their help in the organisation of this study.
Footnotes
Funding: This study was funded by a grant from the Nominoe Fund and the Rennes CHU.
Conflicts of interest: The authors declare no conflicts of interest.
Data sharing: Anonymised participant data will be made available upon requests directed to the corresponding author. Proposals will be reviewed and approved by the sponsor and investigators on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement. All data will be made available for a minimum of 5 years from the end of the study.
Author contributions: CP, PT, and RG conceived the study. CP, RG, VT and PT contributed to the protocol and design of the study. ET, PGB, SO contributed to the implementation of the study or data collection. CH and VT conducted the ELISA serological assays. CP, AS, RG and PW conducted the statistical analysis. CP and RG contributed to the preparation of the report. All authors critically reviewed and approved the final version. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Wang X, Pan Z, Cheng Z. Association between 2019-nCoV transmission and N95 respirator use. J Hosp Infect. 2020;105:104–105. doi: 10.1016/j.jhin.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Team CC-R. Characteristics of health care personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:477–481. doi: 10.15585/mmwr.mm6915e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ECDC. Coronavirus disease 2019 (COVID-19) in the EU/EEA and the UK. Rapid Risk Assessment. eighth update ed 2020.
- 4.Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SPF. Recensement national des cas de COVID-19 chez les professionnels en établissements de soins: Santé Publique, France. Accessed July 20, 2021.https://www.santepubliquefrance.fr/etudes-et-enquetes/recensement-national-des-cas-de-covid-19-chez-les-professionnels-en-etablissements-de-sante
- 6.Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med. 2020;173:120–136. doi: 10.7326/M20-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao J, Fang M, Chen Q, He B. SARS, MERS and COVID-19 among healthcare workers: a narrative review. J Infect Public Health. 2020;13:843–848. doi: 10.1016/j.jiph.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cyril O, Jean-Baptiste B, Elisabeth B, et al. Context of health care workers contamination during the first SARS-CoV-2 epidemic wave in France. Bull Epidemiol Hebdomadaire. 2020;35:690–695. [Google Scholar]
- 9.Richterman A, Meyerowitz EA, Cevik M. Hospital-Acquired SARS-CoV-2 infection: lessons for public health. JAMA. 2020;324:2155–2156. doi: 10.1001/jama.2020.21399. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J Hosp Infect. 2020;105:100–101. doi: 10.1016/j.jhin.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gómez-Ochoa SA, Franco OH, Rojas LZ, et al. COVID-19 in health-care workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2021;190:161–175. doi: 10.1093/aje/kwaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charpentier C, Ichou H, Damond F, et al. Performance evaluation of two SARS-CoV-2 IgG/IgM rapid tests (Covid-Presto and NG-Test) and one IgG automated immunoassay (Abbott) J Clin Virol. 2020;132 doi: 10.1016/j.jcv.2020.104618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garlantezec R, Heslan C, Tadie E, Tattevin P, Thibault V, Paris C. A lateral flow immunoassay test performance in SARS-CoV-2 seroprevalence surveys: a validation study among healthcare workers. Emerg Microbes Infect. 2020;9:2547–2549. doi: 10.1080/22221751.2020.1852893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shields A, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75:1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moscola J, Sembajwe G, Jarrett M, et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324:893–895. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai X, Wang M, Qin C, et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lentz RJ, Colt H, Chen H, et al. Assessing coronavirus disease 2019 (COVID-19) transmission to healthcare personnel: the global ACT-HCP case-control study. Infect Control Hosp Epidemiol. 2020;42:381–387. doi: 10.1017/ice.2020.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iversen K, Bundgaard H, Hasselbalch RB, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20:1401–1408. doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324:195–197. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah ASV, Wood R, Gribben C, et al. Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: nationwide linkage cohort study. BMJ. 2020;371:m3582. doi: 10.1136/bmj.m3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verbeek JH, Rajamaki B, Ijaz S, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2020;4 doi: 10.1002/14651858.CD011621.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raboud J, Shigayeva A, McGeer A, et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto. Canada. PLoS One. 2010;5:e10717. doi: 10.1371/journal.pone.0010717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scales DC, Green K, Chan AK, et al. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis. 2003;9:1205–1210. doi: 10.3201/eid0910.030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds MG, Anh BH, Thu VH, et al. Factors associated with nosocomial SARS-CoV transmission among healthcare workers in Hanoi, Vietnam, 2003. BMC Public Health. 2006;6:207. doi: 10.1186/1471-2458-6-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant JJ, Wilmore SMS, McCann NS, et al. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect Control Hosp Epidemiol. 2021;42:212–214. doi: 10.1017/ice.2020.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr Opin Infect Dis. 2019;32:372–379. doi: 10.1097/QCO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 28.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treibel TA, Manisty C, Burton M, et al. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet. 2020;395:1608–1610. doi: 10.1016/S0140-6736(20)31100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sempos CT, Tian L. Adjusting coronavirus prevalence estimates for laboratory test kit error. Am J Epidemiol. 2021;190:109–115. doi: 10.1093/aje/kwaa174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumleben N, Bhopal R, Czypionka T, et al. Test, test, test for COVID-19 antibodies: the importance of sensitivity, specificity and predictive powers. Public Health. 2020;185:88–90. doi: 10.1016/j.puhe.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]