Abstract
Background and aims
While the higher prevalence of diabetes mellitus (DM) at younger age in Indonesia might contribute to the relatively higher COVID-19 mortality rate in Indonesia, there were currently no available evidence nor specific policy in terms of COVID-19 prevention and management among DM patients. We aimed to find out the association between diagnosed diabetes mellitus (DM) with COVID-19 mortality in Indonesia.
Methods
We performed a retrospective cohort study using Jakarta Province’s COVID-19 epidemiological registry within the first 6 months of the pandemic. All COVID-19 confirmed patients, aged >15 years with known DM status were included. Patients were assessed for their clinical symptoms and mortality outcome based on their DM status. A multivariate Cox-regression test was performed to obtain the relative risk (RR) of COVID-19 mortality in the diagnosed DM group.
Results
Of 20,481 patients with COVID-19, 705 (3.4%) had DM. COVID-19 mortality rate in DM group was 21.28%, significantly higher compared to 2.77% mortality in the non-DM group [adjusted RR 1.98 (CI 95% 1.57–2.51), p < 0.001]. In addition, COVID-19 patients with DM generally developed more symptoms.
Conclusions
DM is associated not only with development of more COVID-19 clinical symptoms, but also with a higher risk of COVID-19 mortality. This finding may provide a basis for future policy regarding COVID-19 prevention and management among diabetes patients in Indonesia.
Keywords: Clinical symptoms, COVID-19, Diabetes mellitus, Indonesia, Mortality
1. Introduction
Indonesia, as the fourth most populous country in the world, has struggled with a huge number of COVID-19 cases during the more-than-one-year pandemic. The total cases have so far reached more than 1.5 million as of April 2021 [1], placing Indonesia as one of the Asian countries with the highest number of COVID-19 cases [[1], [2], [3]]. The mortality rate of COVID-19 in Indonesia has been reported to be slightly higher than the worldwide estimate [1], despite the relatively younger population structure. This, in part, might be contributed by the relatively higher prevalence of cardiometabolic diseases, including diabetes mellitus (DM), at younger age group [[4], [5], [6], [7], [8]].
Previous studies highlighted that those with cardiometabolic risk factors such as diabetes mellitus (DM) have been associated with the worse clinical manifestation and higher mortality in COVID-19 [[9], [10], [11]]. The chronic low-grade inflammation state in people with DM set the stage for further elevations of inflammatory cytokines in COVID-19. Furthermore, immune dysregulation in DM impairs the host’s ability to combat the disease, providing these populations with poorer infection outcomes [12,13]. However, despite overwhelming evidence on the association between DM and worse COVID-19 outcomes, evidence from Asian countries outside China, especially Indonesia is lacking [14,15].
This lack of evidence itself might as well contribute to the lack of initiatives to provide prioritization of COVID-19 prevention and care among those with DM in Indonesia. Therefore, our study aims to describe the association between diagnosed DM with COVID-19 mortality from the Jakarta area, the capital city of Indonesia, during the first 6 months of the COVID-19 pandemic. In addition, we also compare the clinical symptoms between those with and without DM. Evidence reported in our study will not only enrich the available evidence on the association between DM and COVID-19 mortality in Asian and low to middle income countries, but may also set a reasonable basis for future policy in the terms of COVID-19 prevention strategies, including vaccination, while also providing insights on the difference on clinical symptoms for COVID-19 early detection and care among DM patient.
2. Methods
Our study was a retrospective cohort study which included all confirmed case of COVID-19 from the Jakarta province area from 2nd March 2020, the first COVID-19 case in Indonesia, until 31st August 2020. Subjects aged 15 years old or more and without any missing data on previous DM diagnosis status were included in the study. Ethical approval was obtained from the Research Ethics Committee of the Faculty of Medicine, Universitas Indonesia with approval number KET-821/UN.2.F1/ETIK/PPM.00.02/2020.
2.1. Data collection
We reviewed the Epidemiological Surveillance (ES) form of all COVID-19 patients in the Jakarta province area collected by the Jakarta Provincial Health Department. ES form was filled by the attending doctors of the COVID-19 patients from all health facilities, including all primary health care, public and private hospitals. The ES form consists of questions related to the patient’s demographic and clinical characteristics, including signs and symptoms, comorbidities, and mortality outcomes. Diagnosis of COVID-19 cases was confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) of the oro- and/or naso-pharyngeal swab specimen. Patients were classified as having DM when DM was present in the history profile and/or received antidiabetic medicine(s) before COVID-19 diagnosis. All patients not meeting these criteria were included in the non-DM group. Other comorbidities such as hypertension, heart disease, chronic kidney disease, and chronic liver disease were diagnosed based on the history profile and/or any treatment for each respective disease given before COVID-19 diagnosis.
2.2. Study outcome
The primary endpoint was all-cause mortality. All death that occurred after the diagnosis of COVID-19 was considered the consequence of the COVID-19 infection.
2.3. Statistical analysis
Comparison between two groups was analyzed using Chi-Square test for categorical data and unpaired T-test for numerical data. As age, sex, and other comorbidities were reported to affect COVID-19 mortality in many previous studies, they were considered as confounding variables in this study. Thus, a multivariate Cox-regression test was performed to obtain the relative risk (RR) of diagnosed DM with COVID-19 mortality and was adjusted for age, sex, and other comorbidities. All data analysis was performed using SPSS version 25.
3. Results
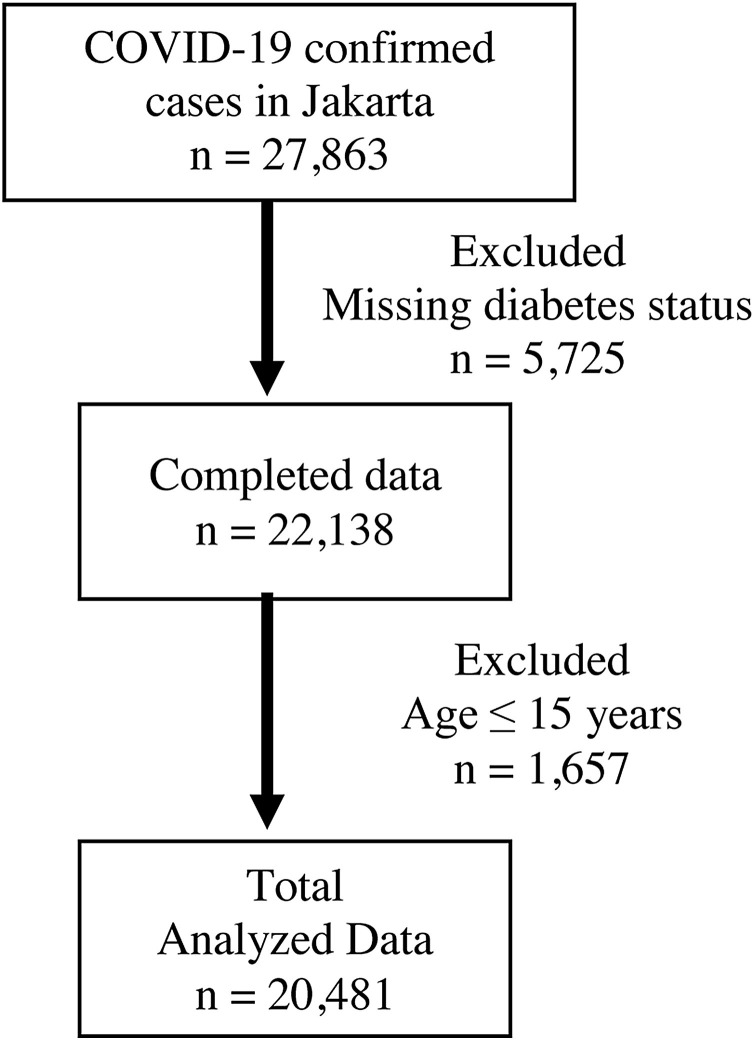
A total of 27,863 COVID-19 patients were registered during the study period. After exclusion of subjects <15 years old and missing diabetes diagnosis status, 20,481 subjects were included for analysis (Fig. 1 ).
Fig. 1.
Consort diagram of study subjects inclusion.
The baseline characteristics of our study subjects are summarized in Table 1 . Diabetes diagnosis was observed in 705 (3.44%) patients. They were generally older and with more comorbidities than the non-DM group. Moreover, COVID-DM patients were also more likely to have symptoms than the non-DM group, notably fever, cough, dyspnea, nausea/vomitus, and pneumonia.
Table 1.
Baseline characteristics of COVID-19 patient in Jakarta.
| Characteristics | Total (n = 20,481) | DM (n = 705) | Non-DM (n = 19,776) | p-Value |
|---|---|---|---|---|
| Age (mean, years) | 41.8 (16.8) | 57 (14.5) | 41 (16.6) | <0.001 |
| Women (n, %) | 9817 (47.94) | 328 (46.52) | 9489 (47.99) | 0.23 |
| Hypertension (n, %) | 1189 (5.82) | 356 (50.5) | 833 (4.2) | <0.001 |
| Heart disease (n, %) | 515 (2.5) | 168 (23.9) | 347 (1.8) | <0.001 |
| Chronic kidney disease (n, %) | 152 (0.74) | 89 (12.6) | 63 (0.3) | <0.001 |
| Chronic liver disease (n, %) | 54 (0.26) | 37 (5.3) | 17 (0.1) | <0.001 |
| History of fever (n, %) | 2417 (11.80) | 349 (50.07) | 2068 (10.48) | <0.001 |
| Cough (n, %) | 3181 (15.53) | 459 (65.38) | 2722 (13.78) | <0.001 |
| Runny nose (n, %) | 1234 (6.02) | 144 (20.51) | 1090 (5.52) | <0.001 |
| Sore throat (n, %) | 1187 (5.79) | 170 (24.22) | 1017 (5.15) | <0.001 |
| Dyspnea (n, %) | 1516 (7.40) | 335 (47.65) | 1181 (5.98) | <0.001 |
| Shivering (n, %) | 575 (2.80) | 120 (17.39) | 455 (2.31) | <0.001 |
| Headache (n, %) | 1369 (6.68) | 225 (32.42) | 1144 (5.80) | <0.001 |
| Muscle pain (n, %) | 938 (4.58) | 158 (22.80) | 780 (3.95) | <0.001 |
| Nausea and vomitus (n, %) | 1263 (6.20) | 277 (40.38) | 986 (5.01) | <0.001 |
| Abdominal pain (n, %) | 594 (2.90) | 137 (19.74) | 457 (2.32) | <0.001 |
| Diarrhea (n, %) | 436 (2.13) | 106 (15.30) | 330 (1.67) | <0.001 |
| Pneumonia (n, %) | 1670 (8.2) | 379 (54.45) | 1291 (6.55) | <0.001 |
Comparison between two groups was analyzed using Chi Square test for categorical data and unpaired T-test for numerical data.
While the mortality rate for COVID-19 in the overall population of this study was 3.41%, for those with DM it was 21.28%. The presence of DM was significantly associated with increased mortality in COVID-19 patients [unadjusted RR and 95% confidence interval (CI): 7.67 (6.51–9.04)] (Table 2 ). In addition, after adjustment for age, sex, and other comorbidities, DM was associated with almost twice times higher mortality in COVID-19 patients compared to non-DM (Table 3 ).
Table 2.
The effect of DM on COVID-19 patient’s mortality.
| Variable | Mortality |
Total | p-Value | RR (95% CI) | |
|---|---|---|---|---|---|
| Death | Survive | ||||
| DM | 150 (21.28) | 555 (78.72) | 705 (3.44) | <0.001* | 7.67 (6.51–9.04) |
| Non-DM | 548 (2.77) | 19,228 (97.23) | 19,776 (96.56) | ||
| Total | 698 (3.41) | 19,783 (96.59) | 20,481 (100) | ||
p Value <0.05, Chi-Square test.
Table 3.
The effect of DM on COVID-19 patient’s mortality adjusted and compared to other comorbidities.
| Variable | SE | p-Value | Adjusted RR (95% CI) |
|---|---|---|---|
| DM | 0.120 | <0.001* | 1.98 (157–2.51) |
| Hypertension | 0.109 | <0.001* | 2.32 (1.87–2.87) |
| Heart disease | 0.131 | <0.001* | 1.61 (1.24–2.08) |
| Chronic kidney disease | 0.187 | <0.001* | 2.02 (1.40–2.91) |
| Chronic liver disease | 0.412 | 0.602 | 0.81 (0.36–1.81) |
p Value <0.05, multivariate Cox regression test, data is adjusted by sex and age.
4. Discussion
Our study confirmed previous reports that DM was independently associated with a significantly higher COVID-19 mortality. In addition, clinical symptoms of COVID-19 were consistently more observed in those with DM.
The increased mortality risk in COVID-DM patients by two fold observed in our study was similar to the data shown by previous meta-analysis [11]. There are several mechanisms on how DM could increase mortality risk in COVID-19 patients. As it is shown in this study, the majority of DM patients in Indonesia has chronic diabetic complications and cardiometabolic comorbidities, which in part is due to the higher proportion of DM patients diagnosed at a late stage [[6], [7], [8]]. However, despite the strong attenuation after the adjustment for important cardiometabolic comorbidities, the association between DM and COVID-19 mortality was still significant, suggesting other pathways might play a role. Several pathways have been reported to play a role: increased expression of angiotensin-converting enzyme-2 (ACE-2) receptor, aging cells, a higher pro-inflammatory condition, and impaired T-cell function and antibody production [11].
It is important to note that our study also observed that patients with DM were more likely to present with clinical symptoms compared to non-DM patients. These findings were in line with previous study which shown that DM increases the risk for severe COVID-19 infection [9,11,16,17]. However, our findings were in contrast with previous study reported similar symptoms of COVID-19 with pneumonia for both DM and non-DM group [18]. This difference might be related with the difference in the study population. Our study analyzed the data from a centralized COVID-19 registry, integrating data from contact tracing at community, primary health care facilities, and hospital. It also included asymptomatic individuals and may thus better represent the COVID-19 cases as a whole.
Our study could not provide a direct answer whether DM was associated with a higher risk to contract COVID-19 infection. If anything, the prevalence of DM diagnosis among COVID-19 patients (3.4%) observed in our study was slightly higher compared to the DM prevalence in general population of the Jakarta province area (2.6%) [5]. Other registry based studies from China and United States (US) showed a DM prevalence in COVID-19 patients of 5.3% and 10.9%, respectively [19]. This prevalence in China is lower than the general population, meanwhile in the US, the prevalence is similar to the general population. However, the relatively lower or similar proportion of DM among COVID-19 infected subjects in comparison to those observed in general population might also be contributed by the widely available health information on the risk of COVID-19 on DM patients and that all DM patients should protect themselves more.
Despite having a relatively younger age of population, the mortality rate for COVID-19 in our study was higher in comparison to worldwide mortality rate. In our study, the mortality rate of COVID-19 patients in Jakarta was 3.41%. This number is comparable with India, another highly-populated low- to middle-income country (LMIC), which reported a case fatality rate of COVID-19 as 3.17–3.88% in June 2020 [20,21]. However, other LMIC in South East Asia, such as Myanmar, show a significantly lower mortality rate with only 0.6% thanks to an overall better management strategy for COVID-19 prevention and management [22]. Nit et al. [23] stated only 302 confirmed cases of COVID-19 with no death in Cambodia, another LMIC country in South East Asia with limited health resources. In part, the presence of DM and other cardiometabolic risk factors at a younger age might play a role [7,8]. In addition, a significant portion of subjects with cardiometabolic risk factors in Indonesia were undiagnosed cases, including the case with DM of which more than 70% of DM cases were undiagnosed [8]. Moreover, majority of DM patients in Indonesia failed to reach their glycemic target [6]. These findings might further contribute to the increase mortality rate due to COVID-19 [[24], [25], [26]] by their influence on immune dysfunction [27] and thromboembolic risks [28]. It is also important to mention that other factors, such as the limited experience during early pandemic and limited healthcare resources might also contribute to the increased mortality [4]. These findings may also suggest that the management of COVID-19 in Indonesia still has room for improvement.
Our study is the first study that showed the impact of DM on COVID-19 mortality in Indonesia, a fourth most populous country in the world. The large number of subjects and data of other comorbidities were strong points for this study. Nonetheless, there are some limitations. The COVID-19 epidemiological registry collected from the ES form is the only data currently available to be analyzed. However, as the majority of resources had been allocated for the care of COVID-19 patients, the completeness of the data was lacking. In such, we have no data on whether DM was well or poorly controlled, and no information on the laboratory parameter such as blood glucose, or data on diabetes medication. Important data related with metabolic disorders such as body mass index or obesity was also lacking.
5. Conclusions
In conclusion, our study not only confirmed previous report that DM is independently associated with a higher risk of COVID-19 mortality, but also evidence that COVID-DM patients were also more likely to have clinical symptoms. This result may be used as a basis for setting a policy regarding COVID-19 prevention among DM patient, including the priority of COVID-19 vaccines. In addition, development of acute infection symptoms among DM patients might warrant early and priority access to COVID-19 test and care. Further study is warranted to understand more the inter-relationship between these two double pandemics, especially its short and long-term health and economic impact.
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Worldometer . 2021. Coronavirus Cases.https://www.worldometers.info/coronavirus/coronavirus-cases/#total-cases Available from: [Google Scholar]
- 2.Setiati S., Azwar M.K. COVID-19 and Indonesia. Acta Med. Indones. 2020;52(1):84–89. [PubMed] [Google Scholar]
- 3.Pemprov DKI Jakarta. Jakarta Tanggap Covid.
- 4.Walker P.G.T., Whittaker C., Watson O.J., Baguelin M., Winskill P., Hamlet A., et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science (80-) 2020;369(6502):413–422. doi: 10.1126/science.abc0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kementerian Kesehatan R.I. Laporan Hasil Riset Kesehatan Dasar (Riskesdas) Indonesia tahun 2018. Riset Kesehatan Dasar. 2018;2018:182–183. [Google Scholar]
- 6.Soewondo P., Ferrario A., Tahapary D.L. Challenges in diabetes management in Indonesia: a literature review. Glob. Health. 2013;9:63–80. doi: 10.1186/1744-8603-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tahapary D.L., Soewondo P. Burden of metabolic diseases in Indonesia: an even more critical issue during COVID-19 pandemic. Med. J. Indones. 2020;29(4):347–349. [Google Scholar]
- 8.Suastika K. The challenges of metabolic disorders in Indonesia: focus on metabolic syndrome, prediabetes, and diabetes. Med. J. Indones. 2020;29(4):350–353. [Google Scholar]
- 9.Abdi A., Jalilian M., Ahmadi P., Vlaisavljevic Z. Diabetes and COVID-19: a systematic review on the current evidences. Diabetes Res. Clin. Pract. 2020;166 doi: 10.1016/j.diabres.2020.108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia — a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman E.L., Savelieff M.G., Hayek S.S., Pennathur S., Kretzler M., Pop-Busui R. Covid-19 and diabetes: a collision and collusion of two diseases. Diabetes. 2020;69(12):2549–2565. doi: 10.2337/dbi20-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berbudi A., Rahmadika N., Tjahjadi A.I., Ruslami R. 2020. Type 2 Diabetes and Its Impact on the Immune System; pp. 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi S.R., Boulton A.J.M. Diabetes and COVID 19 in South-East Asia. Diabetes Res. Clin. Pract. 2020;166 doi: 10.1016/j.diabres.2020.108292. [DOI] [PubMed] [Google Scholar]
- 15.Chanda A. COVID-19 in India: transmission dynamics, epidemiological characteristics, testing, recovery, and effect of weather. Epidemiol. Infect. 2020;148(E182):1–10. doi: 10.1017/S0950268820001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pal R., Bhadada S.K. COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:513–517. doi: 10.1016/j.dsx.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peric S., Stulnig T.M. Diabetes and COVID-19: disease—management—people. Wien. Klin. Wochenschr. 2020;132(13–14):356–361. doi: 10.1007/s00508-020-01672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G., Deng Q., Feng J., Li F., Xiong N., He Q. Clinical characteristics of diabetic patients with COVID-19. J. Diabetes Res. 2020;2020 doi: 10.1155/2020/1652403. 1652403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh A.K., Gupta R., Ghosh A., Misra A. Diabetes in COVID-19: pravalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain K.V., Iyengar K., Vaish A., Vaishya R. Differential mortality in COVID-19 patients from India and western countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:1037–1041. doi: 10.1016/j.dsx.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asirvatham E.S., Lakshmanan J., Sarman C.J., Joy M. Demystifying the varying case fatality rates (CFR) of COVID-19 in India: lessons learned and future directions. J. Infect. Dev. 2020;14(10):1128–1135. doi: 10.3855/jidc.13340. [DOI] [PubMed] [Google Scholar]
- 22.Lin K.S., Win K.H.N., Khine T., Lei S.L., Zaw K.K., Oo W.M. The characteristics and trend of COVID-19 outbreak in Myanmar: lessons from a developing country. Asia Pac. J. Public Health. 2021;3(2–3):311–313. doi: 10.1177/1010539521993696. [DOI] [PubMed] [Google Scholar]
- 23.Nit B., Samy A.L., Tan S.L., Vory S., Lim Y., Nugraha R.R., et al. Understanding the slow COVID-19 trajectory of Cambodia. Public Health Pract. 2021;2(January) doi: 10.1016/j.puhip.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bode B., Garrett V., Messler J., Mcfarland R., Crowe J., Booth R., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 2020:1–9. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sardu C., Onofrio N.D., Balestrieri M.L., Barbieri M., Rizzo M.R., Messina V., et al. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43(July):1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J., Meng W. COVID- 19 and diabetes: the contributions of hyperglycemia. J. Mol. Cell Biol. 2020 doi: 10.1093/jmcb/mjaa054. mjaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daryabor G., Atashzar M.R., Kabelitz D., Meri S., Cari L. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front. Immunol. 2020;11:1582. doi: 10.3389/fimmu.2020.01582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinayagam S., Sattu K. SARS-CoV-2 and coagulation disorders in different organs. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118431. [DOI] [PMC free article] [PubMed] [Google Scholar]