Figure 1.
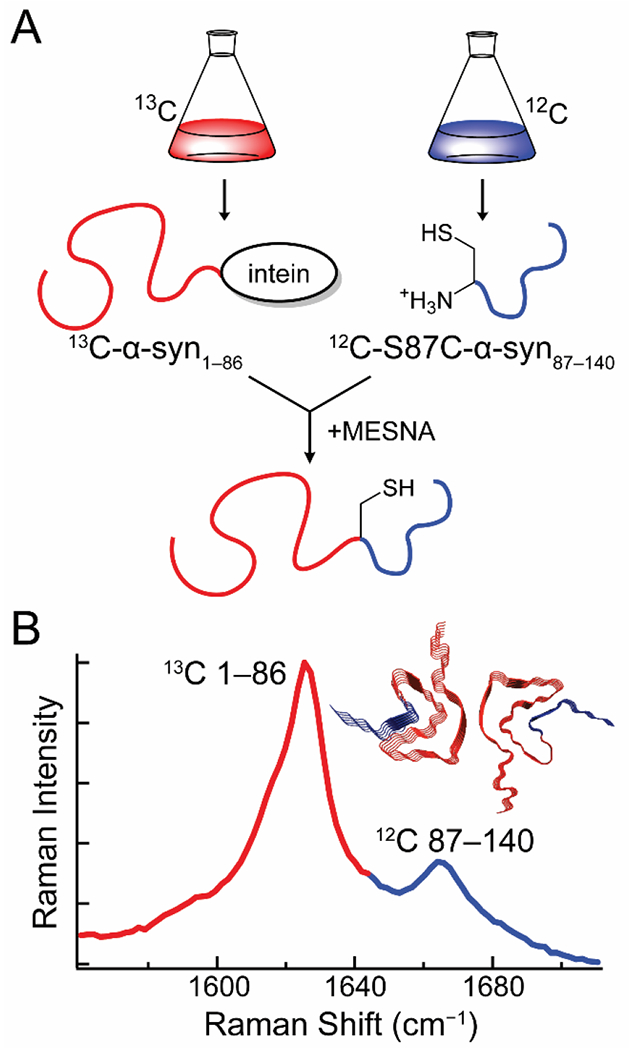
Expressed-protein ligation enables the creation of S87C-α-syn segmentally labeled with 13C and 12C at residues 1–86 and 87–140, respectively. (A) α-Syn1–86 is expressed with a C-terminal intein-tag in 13C-media, while S87C-α-syn is expressed in natural isotope abundance media. A one-pot reaction is carried out where autocleavage of the intein-tag in the presence of 2-mercaptoethanesulfonate (MESNA) leads to covalent ligation of the N-and C-terminal segments of the protein. (B) The 13C-and 12C-labeled segments of the fibrils produce two amide-I bands, enabling independent analysis of their secondary structures. (Inset) Fibril structure of α-syn with the 13C-and 12C-labeled fragments colored red and blue, respectively. Data originally published in [29].
