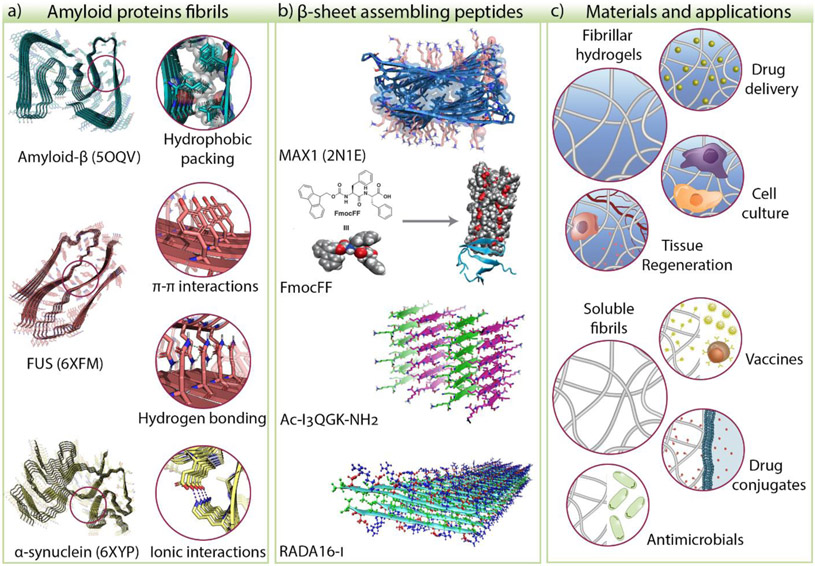
Figure 1.
a) The common structural features of amyloid proteins such as hydrophobic packing, π-π, hydrogen bonding, and ionic interactions have been used to develop β-sheet assembling peptides. b) Examples of peptide systems that rely on these features are MAX1, diphenylalanine derivatives, Ac-I3QGK-NH2, and RADA16-I, to name a few. c) Self-assembling peptides form materials such as fibrillar hydrogels and soluble fibrils used in biomedical applications. The Protein Data Bank (PDB) ID of amyloid-β [32], FUS [57], α-synuclein [39], and MAX1 [53] are shown in parenthesis. The other peptide structures are models or representations of proposed structures. FmocFF model adapted from [58]. © 2019, Ji et al. under CC BY license. Ac-I3QGK-NH2 adapted from [59] © Wang et al. under CC BY 4.0 license. RADA16-I structure image adapted from [54] © 2013, American Chemical Society.