Abstract
The Notch signaling pathway is conserved among mammalian species and controls proliferation, differentiation, and cell death in many organs throughout the body including the reproductive tract. Notch signaling plays critical roles in the development and function of both the male and female reproductive systems. Specifically, within the female reproductive tract, Notch signaling is hormone regulated and mediates key reproductive events important for ovarian and uterine function. In this review, we highlight the tissues that express Notch receptors, ligands, and downstream effectors and distinguish how these molecules regulate reproductive function in male and female mice, non-human primates, and humans. Finally, we describe some of the aberrations in Notch signaling in female reproductive pathologies and identify opportunities for future investigation.
Keywords: Notch signaling, reproduction, gynecological disease
Notch: Regulator of Reproduction
The Notch signaling pathway (Box 1) is ubiquitous throughout all mammalian species and regulates proliferation, differentiation, cell fate and cell death in many tissues[1]. Briefly, in mammals this pathway consists of four cell surface NOTCH receptors (NOTCH1–4) which bind Delta-like (DLL1, DLL3, or DLL4) or Serrate-like (JAG1, JAG2) ligands in a juxtracrine manner resulting in cleavage of the NOTCH receptor and transcriptional activation of target genes (Box 1)[2]. The Notch signaling pathway regulates many facets of embryonic, neural, and vascular development, and its dysregulation contributes to a range of cancers and pathologies[3, 4]. Our primary focus in this review is the Notch signaling pathway’s regulation of reproductive physiology[5]. We will outline the diverse roles of Notch signaling in female and male reproductive development and function, show how this pathway contributes to female reproductive pathologies, and suggest future directions of focus to uncover the mechanisms that regulate Notch signaling.
Box 1. Notch Signaling: An Overview.
The Notch signaling pathway is a juxtracrine signaling pathway which involves four NOTCH receptors (1, 2, 3, and 4) that each contain an extracellular domain (ECD) connected via a calcium dependent, noncovalent bond to the joint transmembrane (TM) and intracellular (IC) domains. These receptors are heterodimers that are activated when a neighboring cell presents one of the delta-like (DLL1, DLL3, and DLL4) or serrate-like (JAG1 and JAG2) ligands, leading to a series of cleavage steps. Prior to engaging with a ligand, NOTCH receptors must undergo preprocessing. The NOTCH receptors are first fucosylated by POFUT1 in the endoplasmic reticulum[66]. The final mature receptor is produced after cleavage by a furin convertase at the first cleavage site known as S1. The fringe glycosyltransferases, Lunatic Fringe, Manic Fringe and Radical Fringe, can then glycosylate the NOTCH receptors in the golgi mediating receptor-ligand interactions [67]. The receptor is then transported to the cell membrane through the secretory pathway where it can interact with a ligand presenting cell and induce the Notch signaling cascade. Once the receptor has been activated by ligand, a disintegrin and metalloprotease (ADAM10 or ADAM17) cleaves the ECD from the joint TM and ICD at the S2 cleavage site. Subsequently, the ICD is cleaved from the TM by the γ-secretase complex at the S3 and S4 cleavage sites allowing the NOTCH intracellular domain (NICD) to translocate to the nucleus where it binds to its transcriptional effector, recombination signal binding protein for immunoglobulin kappa J region (RBPj) and other transcriptional cofactors to initiate transcriptional activation of NOTCH target genes such as myc, cyclin D3, and the Hairy and Enhancer of split (HES) family of genes[66]. A more detailed view of NOTCH receptor structure as well as detailed molecular mechanisms can be found in previously published literature[66, 68].
Hormonal Regulation of Notch signaling
Despite the physiological importance of Notch signaling, there remain significant gaps in our knowledge regarding its regulation and functional diversity (Box 2). Major gaps currently include factors upstream of receptor and ligand presentation as well as tissue-specific signaling mechanisms. The governing hormones within the female reproductive tract are estradiol (E2), progesterone (P4), and human chorionic gonadotropin (hCG). These hormones regulate processes that are integral to fertility maintenance, like ovarian and uterine development, and the ovarian and menstrual cycles (Box 4). Thus, the Notch signaling pathway, which we suggest is an arbiter of reproductive function, could be regulated by these essential hormones. In mice, Notch signaling activation mimics P4 levels throughout the estrous cycle indicating that P4 signaling may contribute to cycle specific regulation of Notch signaling activation[6]. Transcriptional levels of receptors: Notch1-4, ligands: Dll4, Jagged 1 and 2, and target genes: Hes1, Hes5, and Nrarp increase in the mouse uterus throughout the estrous cycle indicating that the Notch signaling pathway activity may increase as the estrous cycle progresses[6]. In the Olive baboon, Papio anubis, Notch1 expression is low in the endometrium during the proliferative phase of the menstrual cycle (Box 4) followed by an increase in glandular expression of Notch1 during the secretory phase[7]. In addition, Notch1 expression is positively regulated by hCG[7, 8]. Following administration of hCG, Notch1 increases in the stromal compartment of the endometrium providing a role for embryonic signal-mediated regulation of Notch signaling[7]. Additionally, hCG induces NOTCH1 expression in human stromal cells in vitro[7]. In a clinical trial to determine the effect of hCG infusion on the human endometrium during the period of implantation, increased glandular and stromal protein levels of NOTCH1 were noted without observable changes in mRNA expression, suggesting that hCG may induce post-translational modification of NOTCH[9]. To further investigate how hCG regulates Notch1 in the endometrial environment, human uterine fibroblasts (HuFs) were treated with P4 and hCG, which resulted in cleavage of NOTCH1 at the primary γ-secretase cleavage site, valine residue 1744, suggesting that hCG acts to induce NOTCH1 signaling activation in stromal cells[7]. E2 also significantly affects Notch signaling in several systems. Treatment of human umbilical vein endothelial cells with E2 leads to increased activation of NOTCH1 and NOTCH4 and decreased NOTCH2 expression showing that E2 modulates Notch signaling activation in some cellular contexts[10]. Conversely, in breast cancer cell lines, E2 treatment reduces Notch signaling activation by causing accumulations of full-length membrane bound NOTCH1[11]. Additionally, N1ICD can stimulate estrogen receptor α (ER) dependent transcription in the absence of estradiol in breast cancer cells by recruiting coactivators to ER-responsive elements[12]. E2-regulated NOTCH signaling has also been described in various tissues throughout the reproductive tract. Ishikawa cells, an endometrial carcinoma cell line, exhibit increased availability of NOTCH1 protein and mRNA with E2 administration[13]. In addition, an in vitro study of human fallopian tube epithelial cells showed that E2 and P4 induced an increase in mRNA expression of a NOTCH target gene, Hes1[14]. These studies collectively indicate that there may be tissue specific and even cell type specific hormonal regulation of Notch signaling at multiple levels.
Box 2. Notch Signaling Regulation.
The known upstream regulators of NOTCH receptors are the Fringe glycosyltransferases that modify NOTCH receptors by the addition of N-acetylglucosamine to fucosylated EGF repeats within the extracellular domain[69]. These modifications help to specify ligand interactions with NOTCH receptors at the cell surface[66]. The NOTCH intracellular domain (NICD) is also regulated after full-length NOTCH cleavage. During development, CDK1 and CDK2 phosphorylate NICD controlling its half-life. Phosphorylated NICD is rapidly targeted for ubiquitination and degradation, limiting signal propagation[70]. A novel stabilizing factor of NICD in breast cancer cells, ubiquitin-specific protease 8, deubiquitylates NICD, which increases NICD cytoplasmic stability[71]. There may be other undiscovered mechanisms regulating the half-life of the NOTCH intracellular domains[71]. NOTCH receptors are also targeted for lysosomal degradation by E3 ubiquitin ligases[66].
Box 4. Reproductive Events in the Female: A Brief Overview.
The Menstrual Cycle is a 28-day cycle which governs ovarian and endometrial cyclicity in humans. Ovarian hormones regulate changes in the endometrium inducing three independent phases, the menstrual phase (days 0–5), the proliferative phase (days 6–13), ovulation (day 14), and the secretory phase (days 15–28). The endometrium itself is composed of two layers, the functionalis which is responsive to ovarian steroids and is shed with each cycle during menstruation, and the basalis which serves as the source of endometrial regeneration[73]. E2 levels dominate in the proliferative phase, which regenerates the functionalis layer after menstrual shedding. Ovulation occurs around day 14 of the cycle and is caused by spikes in estrogen (E2), follicle stimulating hormone (FSH), and luteinizing hormone (LH). The secretory phase follows, which is characterized by increasing P4 and decreasing E2 levels, more functionalis growth, and the initiation of stromal cell decidualization. If an oocyte is fertilized, the endometrium prepares for implantation during the window of uterine receptivity (days 20–24)[5]. If an oocyte is not fertilized, a lack of P4 support triggers menstruation and the beginning of a new cycle[23].
The Estrous Cycle occurs over four days in the mouse and involves 4 distinct stages: proestrus, estrus, metestrus, and diestrus. Proestrus is characterized by high FSH, LH, E2, and prolactin (PRL) secretion Estrus is hormonally characterized by peaks in PRL and FSH levels and results in ovulation. Metestrus is characterized by an increase in P4 levels while diestrus serves as the P4 dominant phase[74]. Unlike primates, mice do not menstruate and only undergo a decidualization reaction in response to an implanting embryo.
Decidualization is the terminal differentiation process of stromal cells wherein they transform from mesenchymal to epithelioid-like cells termed decidual cells. The stromal cells of the endometrium begin to decidualize in response to elevated P4 levels during the secretory phase in humans[75]. After successful blastocyst implantation, the decidua forms, which is a highly vascularized tissue composed of decidual stromal cells, leukocytes, hematopoietic cells and uterine natural killer cells [76]. The decidua’s function is to support and aid in the establishment of pregnancy[5, 77].
Implantation Embryo implantation involves several major steps which include apposition, adhesion, and invasion of the embryo into the maternal uterine tissue. This process initiates the decidualization reaction in mice, but implantation induces further decidualization in humans[73]. The detailed processes of decidualization and implantation and their key players have been reviewed elsewhere [5, 73, 75, 77].
Placentation and the placental structure vary considerably between different species. Humans, nonhuman primates, and mice all have hemochorial placentas, meaning that villi lined by trophoblasts come into direct contact with maternal blood[78]. The placenta functions as the nutrient and oxygen transporter from mother to fetus during pregnancy and therefore serves important life sustaining roles throughout pregnancy. Placental development in mice and humans has been reviewed elsewhere[78].
Notch Signaling in Reproductive Physiology
Male Reproduction
The Notch signaling pathways plays notable roles throughout the male reproductive system (Key Figure 2). Notch1 and its ligand Jag2 are expressed in the rat testis during spermatocyte development[15]. N1ICD constitutive expression under a mouse mammary tumor virus long terminal repeat (MMTV/Notch1intraΔCys) in male mice results in sterility[16]. The authors verified N1ICD transgene expression in the efferent ducts, epididymis, and vas deferens and total Notch1 was found in both the epididymis and the vas deferens[16]. There were no differences between MMTV/Notch1intraΔCys expressing mice and their wild-type littermates at postnatal days 8 and 21, but adult mice at postnatal day 28 exhibited undifferentiated proximal epididymis, epithelial folding, and absence of spermatozoa in the epididymis, and hyperplasia in the vas deferens[16]. The authors concluded that constitutive expression of the N1ICD resulted blockage of the rete testis-efferent duct interface preventing the migration of sperm, inducing infertility[16]. While this study shows that constitutive activation of Notch signaling is detrimental to male murine fertility, inhibition of Notch signaling also compromises fertility. Treatment of adult male mice with DAPT, a γ-secretase complex inhibitor known to inhibit Notch signal propagation, for an entire cycle of spermatogenesis or for half of a spermatogenesis cycle resulted in abnormal germ cell morphology and increased germ cell apoptosis ultimately disrupting spermatogenesis and resulting in morphologically abnormal sperm, potentially compromising fertility[17]. It is important to note that DAPT, like other γ-secretase inhibitors, is an indirect inhibitor of Notch signaling and that the γ-secretase complex has many receptor targets including NOTCH receptors, amyloid-β precursor protein, ErbB4 and other type I membrane proteins indicating that these aberrations in spermatogenesis may result from the effect of these inhibitors on other signaling pathways (Box 3)[18, 19]. Another study performed in a murine model showed that Notch receptors and ligands are expressed temporally in the testis specifically within the seminiferous tubules in multiple cell types including germ cells, Sertoli and Leydig cells indicating that Notch signaling is active during spermatogenesis, the process that results in the production of mature sperm[20]. In addition to localization within the seminiferous tubules, Notch1, Notch2, Notch3, Dll4, Jag1, Hes1, and Hes2 transcripts are highly expressed in the epididymis and Notch1, Notch2, and Hes5 transcripts are evident in the vas deferens[21]. Notch signaling is active as evidenced by nuclear localization of NICD, suggesting a role for Notch signaling in sperm maturation[21]. Together these studies indicate that careful regulation of Notch signaling is required for male fertility, and that Notch signaling contributes to male reproductive tract development and function and offers areas of future study to investigate the precise mechanisms by which Notch signaling regulates these critical processes.
Key Figure 2: Notch signaling molecule expression in the male reproductive tract.
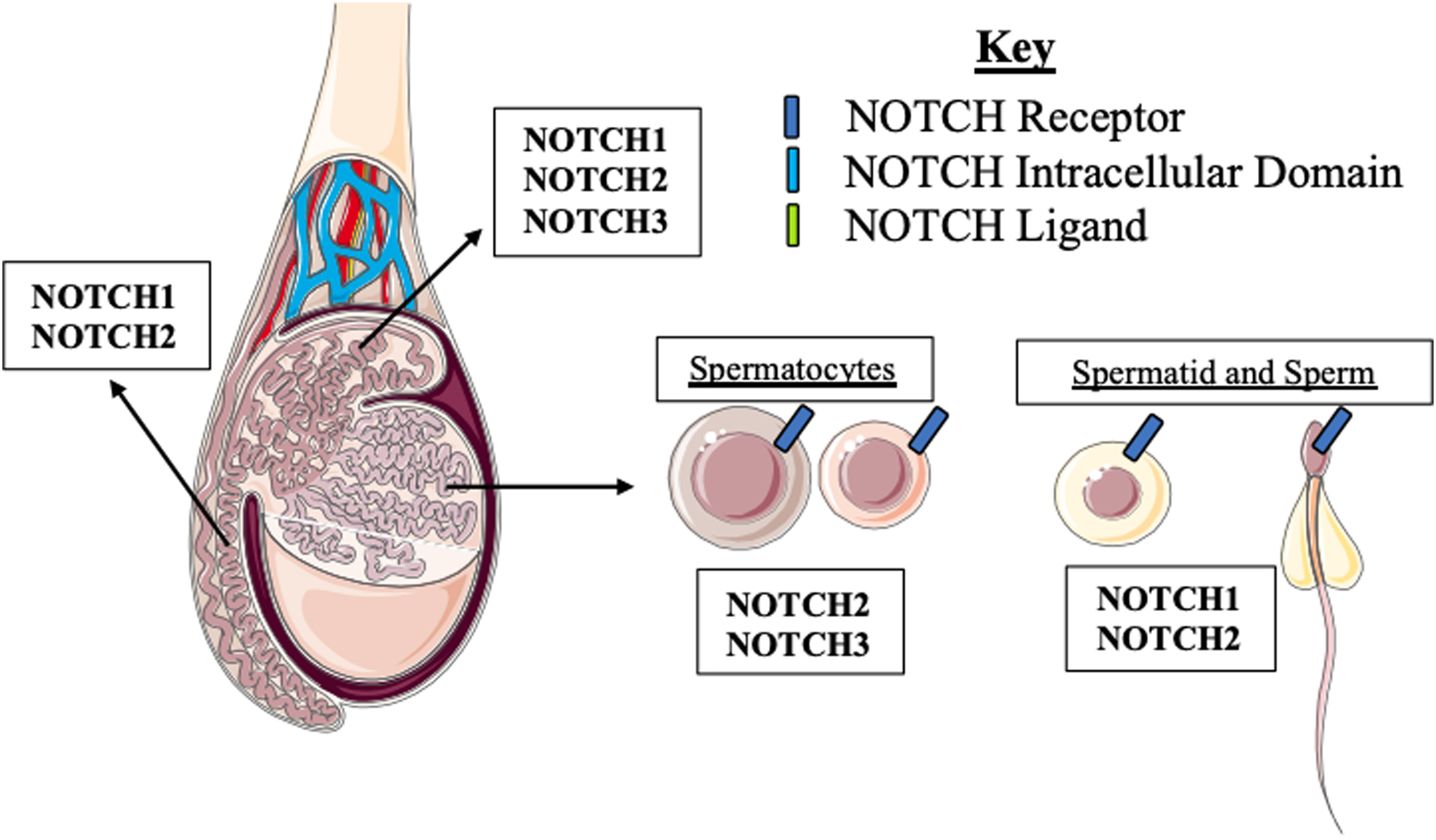
Receptors NOTCH1, 2, and 3 are expressed in the epididymis while NOTCH1 and 2 are the dominant receptors in the vas deferens. In the seminiferous tubules. NOTCH2 and 3 are expressed in spermatocytes while NOTCH1 and 2 are expressed spermatids and mature sperm.
Box 3. Elucidating Notch Signaling.
Studies that investigate Notch signaling in reproduction employ several methods each of which have their own set of advantages and disadvantages. The most common methods include murine knockout and overexpression models that target specific members of the Notch signaling pathway. For instance, a progesterone cre recombinase mediated deletion of the Notch transcriptional effector Rbpj, Notch1, or overexpression of N1ICD is highly specific to cells that express the progesterone receptor including cells of the ovary, endometrium, testis, and mammary gland. These models have greatly contributed to the understanding of Notch signaling in the context of reproduction and are advantageous because they highlight the physiological effects of the Notch signaling pathway in tissue and cell specific manners. In addition to transgenic mouse models, in vitro studies frequently employ recombinant proteins for the Delta-Serrate family of ligands to determine which ligands are involved in a specific system and are advantageous for dissecting precise mechanisms but lack physiological context. In vitro and in vivo studies also utilize pharmacological inhibitors that target the γ-secretase complex to prevent the cleavage of NOTCH receptors. The use of γ-secretase inhibitors like DAPT are fairly nonspecific since the γ-secretase complex targets many type I transmembrane proteins and therefore these results are less informative without confirmation of Notch signaling inhibition through modifications in target gene expression[19]. In addition, novel methods have been developed outside the field of reproduction including fluorescent reporter constructs that allow researchers to investigate interactions between Notch and other pathways of interest such as the Wnt and Hedgehog pathways[72].
Female Reproduction
Notch signaling is critical for male reproductive function and plays equally important roles throughout the female reproductive system (Key Figure 1 and Box 4). Notch signaling components are expressed throughout the female reproductive tract in rodents, baboons, and humans. In mice, Notch signaling components including Notch1-4, Jagged1-2, and Dll4 and target genes Hes1, 2, and 5 are all expressed in the oviduct and within the mouse uterus[6]. It is thought that the Notch signaling pathway contributes to cellular remodeling that occurs throughout the estrous cycle since this pathway functions through cell-to-cell contact and is differentially expressed throughout the estrous cycle[6]. We have shown that Notch1 protein expression varies throughout the menstrual cycle suggesting that Notch signaling may play distinct roles in primates[7]. Histological analyses of the human endometrium have shown a similar pattern of expression throughout the menstrual cycle[22]. The human endometrial glandular epithelium expresses NOTCH1 and NOTCH4 during the proliferative phase, and the glandular epithelium and stroma express NOTCH1, NOTCH4, and JAG1 during the secretory phase indicating that the Notch signaling pathway is most likely to be active during the secretory phase of the menstrual cycle[22].
Key Figure 1: Notch signaling molecule expression in the female reproductive tract.
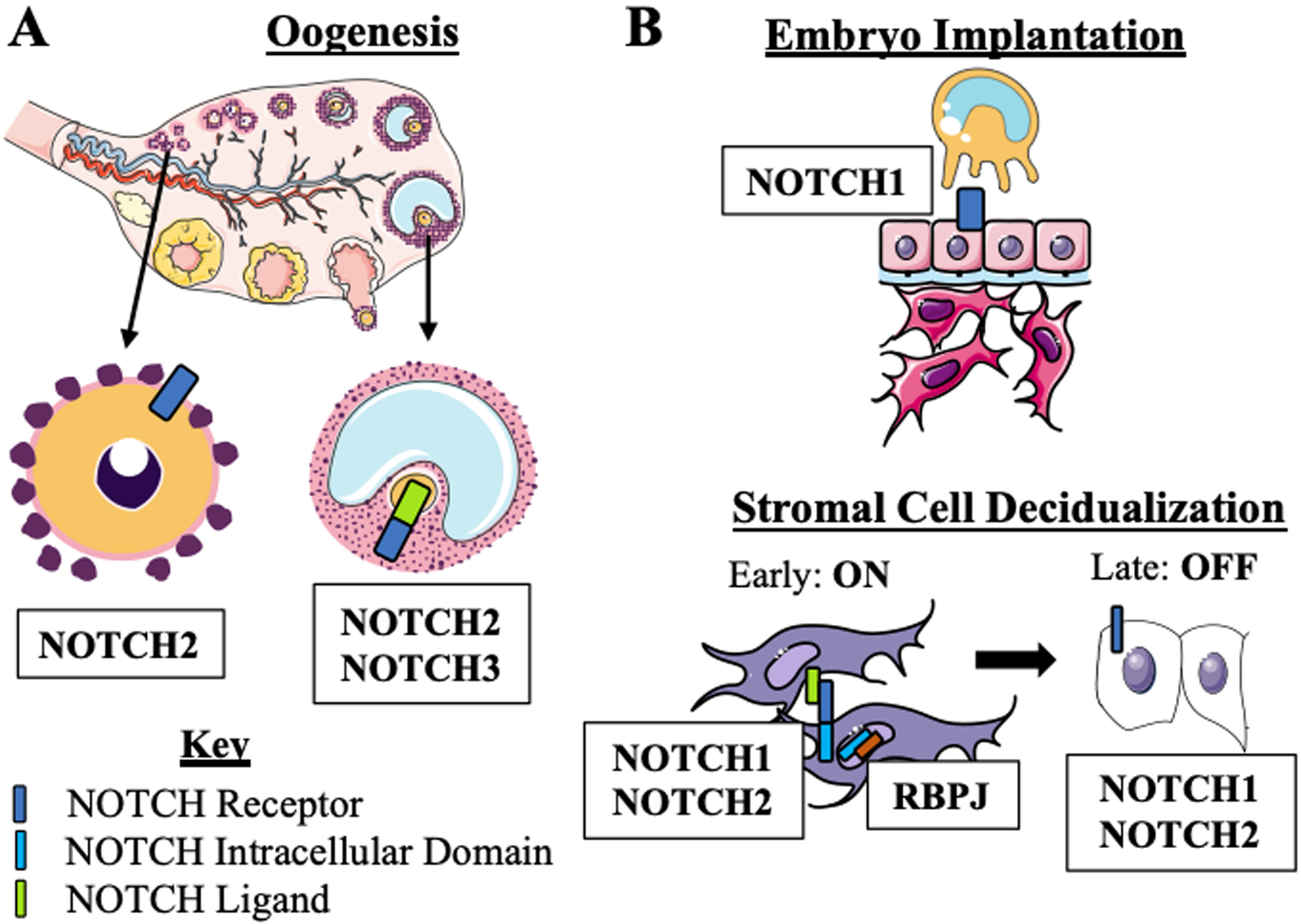
(a) Notch receptors are expressed in ovarian cells. During prenatal and early postnatal ovarian development, NOTCH2 is the dominant receptor expressed by the primordial follicles. Later in oogenesis, NOTCH2 and 3 on the surface of granulosa cells interacts with JAG2 ligand expressed by the oocyte. (b) Notch signaling is active in the endometrium during implantation and decidualization. During the window of implantation, epithelial cells on the surface of the endometrium express NOTCH1 During the initiation of decidualization. NOTCH1 and 2 receptors are active. When the Notch receptor is activated by a ligand on an adjacent cell, a cleavage event occurs that results in the release of the intracellular domain. The ICD then translocates to the nucleus to interact with RBPJ. the Notch signaling transcriptional effector. During late decidualization. NOTCH1 and 2 are expressed but Notch signaling is inactive. Some art elements obtained form Servier Medical Art (http://smart.servier.com) Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
The Notch signaling pathway is clearly active throughout the female reproductive tract but what are its specific roles in these diversely functioning tissues? The ovary contains maturing follicles that are surrounded by granulosa cells, extracellular matrix, and a layer of theca cells. After ovulation, the remaining granulosa and theca cells transform into the luteal cells of the corpus luteum, which provides hormonal support in early pregnancy[23]. Within the ovarian environment, Notch signaling has been shown to play roles in granulosa cell differentiation, luteal cell function, and ovarian steroid hormone production[24]. During embryonic ovarian development, Jag1, Jag2, Notch2 and targets Hes1 and Hey2 are highly expressed in the prenatal ovary[25]. Our understanding of the roles of Notch signaling in the developing murine ovary has evolved from research conducted utilizing conditional knockout mouse lines. Two such models include the Vasacre/+ Jagged1Fl/+ mice that contain a conditional deletion of the Notch ligand Jag1 in their germ line, and Amhr2cre/+ Notch2Fl/+, which conditionally deletes Notch2 in the developing ovary[25]. These models are temporally specific and have allowed researchers to determine the importance of Notch signaling in specific cell types at critical developmental timepoints. The ovaries from both transgenic models contain multioocytic follicles indicating a failure to form correct follicle units prenatally[25]. Additionally, the postnatal follicles in these mice display decreased granulosa cell proliferation and increased apoptosis indicating that Notch signaling is required for granulosa cell survival[25]. Notably, the Jag1 knockout mice are subfertile, and both knockout models show premature reproductive aging suggesting that Notch signaling is the prenatal ovary is required for proper ovary function[25]. Postnatal studies in the ovary conducted in the presence of hCG resulted in increased Jag1 and Notch2 expression indicating that Notch signaling is hormonally regulated in the ovary[26]. Further studies utilizing DAPT, a γ-secretase inhibitor, in organ-cultured ovaries resulted in degenerating oocytes, decreased primordial follicles which were negative for Ki-67, a marker of proliferation, signifying that Notch signaling is integral to oocyte survival and proliferation[27]. Although Notch signaling is most likely an important pathway in the ovary, the γ-secretase complex cleaves many proteins that regulate proliferation. Therefore, these results may also indicate a role for other pathways that promote oocyte survival and proliferation (Box 3)[18]. More recently, evidence of lateral crosstalk of the Notch signaling pathway amongst multiple cell types in the ovary has surfaced[28]. In this study the authors determine that Jag1 in the oocyte activates Notch2 or Notch3 in granulosa cells showing that different cell types communicate via Notch signaling[28]. These studies support that the Notch signaling pathway is integral to maintaining fertility at the level of the ovary through developmental regulation and granulosa cell function.
The endometrium is the primary site of female reproductive function and cyclically responds to ovarian hormones and the presence of an embryo, ultimately providing a hospitable and nourishing environment for an embryo and fetus (Box 4). The endometrium is composed of four major cell types, the epithelium that forms the lumen and branching glands, stromal cells that provide support in the endometrium, various immune cells that regulate maternal-fetal interactions and repair within the endometrium, and endothelial cells that line the spiral arteries, which serve to support the embryo during implantation and in utero development[5]. The cells that make up the endometrium participate in significant crosstalk to encourage reproductive success (Box 4). The cyclical changes of the menstrual cycle, decidualization, implantation, and uterine repair are all processes that are in part regulated by Notch signaling.
Decidualization is the terminal differentiation of endometrial stromal cells that begins during the latter part of the menstrual cycle in humans and non-human primates, and at the time of implantation in mice (Box 4). Decidualization is an important process for the success of pregnancy. Decidualized stromal cells function throughout pregnancy in the maternal decidua, a tissue which forms as the maternal side of the placenta. Notch1 regulates decidualization in mice, nonhuman primates, and humans[7, 29, 30]. Extensive studies in our laboratory provide a role for Notch signaling in endometrial stromal cell decidualization. In HuF cells, knockdown of the NOTCH1 receptor compromises the decidualization response when these cells are treated with decidualization stimuli: E2, the progesterone analogue medroxyprogesterone acetate (MPA), and cAMP (EPC)[7]. In a conditional Notch1 knockout mouse model in the uterus (Pgrcre/+ Notch1Fl/Fl), we noted a decreased decidualization response as measured by uterine wet weight and mRNA expression of decidualization markers Bmp2 and Wnt4[29] and a smaller litter size in the first pregnancy[31]. While these data suggest that Notch1 is required for an appropriate decidualization response both in vitro and in vivo, we sought to investigate the specific mechanisms of these interactions. We generated a uterine N1ICD overexpression mouse model (Pgrcre/+ Rosa26N1ICD/+) to ascertain whether constitutive activation of the Notch1 signaling pathway would affect the decidualization response. We observed that constitutive activation of the Notch1 signaling pathway through N1ICD overexpression compromises decidualization and implantation[32]. We also sought to determine if Rbpj, the transcriptional effector for all four Notch receptors, plays an independent role in the uterus. In an Rbpj conditional knockout mouse model in the uterus (Pgrcre/+ RbpjFl/Fl), we observed a severely compromised decidualization response, as measured by uterine wet weight and mRNA levels of Bmp2 and Wnt4, and compromised uterine repair that resulted in a recurrent pregnancy loss phenotype[33]. In this model, we determined that the decidualization failure occurred as a result of decreased progesterone receptor expression and signaling as well as reduced Slc2a1 expression, a glucose transporter important for stromal cell differentiation[33]. We further characterized the roles of Notch signaling in vitro through Notch1 knockdown in HuF cells followed by EPC treatment to induce decidualization[30]. These data indicate that NOTCH1 knockdown prior to the initiation of decidualization compromises the decidualization response, but if the decidualization reaction has already begun, NOTCH1 knockdown has no effect[30]. These studies indicate that NOTCH1 is an early regulator of decidualization in endometrial stromal cells. Another Notch receptor is also implicated in endometrial stromal cell decidualization. In primary human decidual stromal cells (HDSCs) of early pregnancy, NOTCH2 is the most abundant receptor as determined by immunofluorescence of decidual tissue[34]. The authors then treated isolated HDSCs in vitro with cAMP, E2 and P4; and EPC treatment. There were no alterations in NOTCH2 protein or mRNA expression after 3 and 6 days of treatment but increases in ligands DLL1 and DLL4 were induced with cAMP and EPC treatment showing that Notch ligand availability is decidualization dependent[34]. Furthermore, inhibition of Notch2 expression or activation treatment of HDSCs with subsequent EPC treatment compromised decidualization suggesting that NOTCH2 expression and signaling activation are required for decidualization in HDSCs[34]. While we and others have established a clear role for Notch signaling in the decidualization process, the question that remains is what regulates Notch signaling during decidualization and how is Notch signaling mediating the decidualization response? This remains an open area of research, but one study shows that post-translational modification of the Notch1 receptor may be one mechanism. Fucosylation of Notch1 by poFUT1 increased receptor-ligand binding since we know from previous studies that Notch receptors contain EGF repeats that are glycosylated to promote receptor-ligand interactions[35]. This mechanism permits increased Notch1 signaling activation in endometrial stromal cells, but when poFUT1 is inhibited, fucosylation of Notch1 is decreased leading to a compromised decidualization response[35]. This suggests that fucosylation is an active mechanism of Notch1 signaling regulation in decidualizing endometrial stromal cells[35]. Future directions for the field include discerning mechanisms, either hormonal or otherwise, that are regulating the Notch signaling pathway during the decidualization process and how the Notch signaling mediates the decidualization response.
Implantation is the process whereby a developing embryo enters the uterine cavity and attaches to the endometrium following fertilization (Box 4). Notch signaling and target gene expression play a role throughout the implantation process. During the peri-implantation period, Notch signaling is active in the endothelial cells of the uterus[36]. Endothelial cells within the uterus typically form the vasculature and are critical for nutrient delivery through the uterus to the fetus. In addition, decidualization, implantation, placental development and pregnancy maintenance are all dependent on angiogenesis or the formation of new blood vessels[36]. Notch and vascular endothelial growth factor signaling are well known players in angiogenesis but have only recently been associated with decidual angiogenesis[36]. Notch1, 2, and 4 as well as ligands Dll4 and Jag1 are expressed in murine endothelial cells during the peri-implantation period and there is limited expression of Notch3 and Jag1 in pericytes, vascular peripheral cells, prior to implantation[36]. In addition, Notch1, 4, Dll4 and Jag1 are expressed in decidual endothelial cells after implantation suggesting a role for Notch signaling in pre- and post-implantation angiogenesis[36]. An endothelial post-implantation, pre-placentation Jag1 deletion mouse model exhibits increased Notch1 signaling and increased Dll4 expression, suggesting that Jag1 is not an essential Notch ligand in post-implantation, pre-placentation angiogenesis[37]. Early studies focused on identifying expression of Notch signaling member expression in angiogenesis, therefore functional analyses of Notch signaling in post-implantation angiogenesis are lacking. Notch signaling also plays direct roles in the endometrium during the implantation process. Notch1 receptor and ligands, DLL4 and Jagged1, are expressed on the apical surface of luminal epithelial cells of the mid-secretory endometrium indicating that Notch signaling may aid in blastocyst implantation[38]. Cuman et al reported that blastocyst-conditioned media influences Notch1 and Jag1 expression in epithelial cells in vitro suggesting that blastocysts may modulate uterine receptivity and implantation through mechanisms that alter Notch signaling[39]. In the peri-implantation uterus, Rbpj is expressed in stromal cells in the primary and secondary decidual zones[40]. In an Rbpj uterine conditional deletion mouse model (Pgrcre/+ RbpjFl/Fl), deletion of Rbpj results in altered embryo implantation orientation producing a high rate of miscarriage as evident by many resorbing implantation sites[40]. The deletion of Rbpj also results in increased E2 responsiveness in the endometrial stroma and increased epithelial cell proliferation[40]. The authors noted that Rbpj directly interacts with estrogen receptor α (ER) in a Notch independent manner suggesting that Rbpj may act to regulate E2 signaling in the endometrium[40]. These studies show expression and function of Notch signaling components within the processes of implantation, implantation associated angiogenesis, and maternal-fetal crosstalk of endometrial receptivity.
In addition to the Notch signaling pathway’s role in the uterus throughout the menstrual cycle and in the establishment of pregnancy, the Notch signaling pathway is implicated in placentation[41]. During the initiation of decidualization, Notch signaling is active in endothelial cells of the decidua and in nonvascular decidual cells at embryonic day 8.5 in a mouse model[41]. In the mature murine placenta on embryonic day 12.5, Notch receptors and ligands are expressed in the decidua, junctional zone, and labyrinth in multiple cell types indicating that the Notch signaling components are available during placental development and function[41]. Notch1 signaling is active in proximal cell column trophoblasts and promotes proliferation and cell survival in the mature placenta[42]. In conjunction, Notch2 is the active receptor in the distal cell column of placental villi and functions to promote endovascular invasion and remodeling[42]. Another functional study established the role of Rbpj, the primary NOTCH signaling transcriptional effector, in placental morphogenesis[43]. Lu et al generated several Rbpj systemic, and conditional knockout mouse models that revealed that RBPJ deletion in the placenta compromises chorioallantoic branching and trophoblast differentiation in the ectoplacental zone[43]. These results combined with their other observations indicate that allantoic Rbpj expression targets Vcam1 to aid chorioallantoic fusion which is critical for fetal vessel development in the chorion. In addition, Rbpj is essential for specification of trophoblast cells as it functions with Mash2 to induce trophoblast specific protein α expression[43]. These diverse roles of Rbpj show the ubiquitous importance of Notch signaling and independent action of Rbpj as drivers of placental structure and function.
Aberrant NOTCH Signaling in Gynecologic Pathologies
Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) affects approximately 10–20% of reproductive aged women and is characterized by hormonal dysregulation accompanied by enlarged ovaries with an accumulation of ovarian follicles creating a cyst-like appearance on the surface of the ovary[44]. Notch receptor and ligand expression is aberrant in patients with PCOS including decreased NOTCH1, JAG1, JAG2, and increased NOTCH3 mRNA expression during the window of implantation in endometrial samples (Figure 3) [45]. Protein expression of JAG1 and JAG2 is also decreased in the endometrium of PCOS patients[45]. In addition to altered endometrial expression, Notch signaling is enriched in granulosa cells of patients with PCOS as identified by microarray analysis, and members of this pathway are suggested as potential biomarkers of PCOS[46]. Together these data suggest that Notch signaling may contribute to PCOS pathology and could be utilized to identify patients with PCOS.
Figure 3. Notch receptor and ligand expression aberrations in gynecological pathalogies.
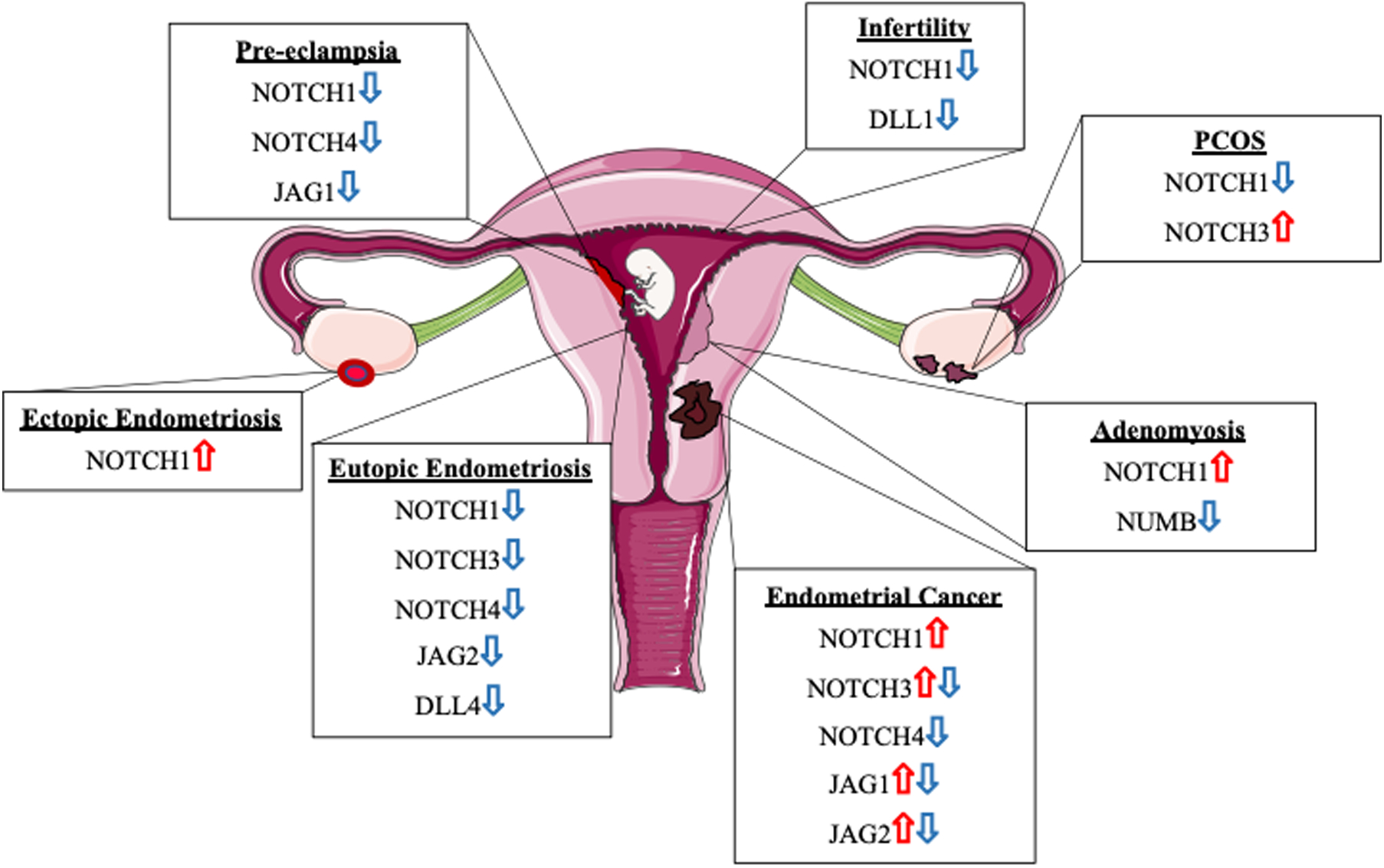
Notch receptors and ligand expression are dysregulated in a myriad of gynecological pathologies including pre-eclampsia. infertility, PCOS, adenomyosis. endometrial cancer, and eutopic and ectopic endometriosis in comparisons against disease-free controls. These expression alterations contribute to infertility and disease pathology by comproimsing decidualization and hormone signaling, inducing recurrent pregnancy loss, increasing epithelial to mesenchymal transition, and contributing to placental defects.
Endometriosis
Endometriosis is an E2 dependent disease characterized by endometrial-like tissue found at ectopic sites, most commonly in the peritoneal cavity[47]. This disease is associated with idiopathic infertility and is a major cause of pelvic pain in women of reproductive age[47]. We have shown that NOTCH1 expression as well as ligands JAG2 and DLL4 and NOTCH1 gene targets NOTCH4, HES5, and HEY1 are significantly decreased in the eutopic (Eu) endometrium (E) of women with endometriosis[30]. In addition, stromal cells isolated from women with endometriosis have reduced NOTCH1 and ligand expression as well as a reduced decidualization response in vitro[30]. We hypothesized based on gene expression profiling, that the reduced Notch1 signaling contributes to a decidualization defect in women with endometriosis by downregulating FOXO1, a molecule that is critical for many reproductive functions including decidualization[30, 48–51]. Another study has corroborated the decrease in NOTCH1 and ligand expression in the EuE of women with endometriosis[45]. In addition, during the window of receptivity the endometrium from women with endometriosis exhibits reduced NOTCH3 expression[45]. Additional histological studies determined the localization of NOTCH receptors and ligands in the endometrium of women with and without endometriosis[52]. In addition to the EuE, the Notch signaling pathways is integral in endometriotic lesion pathogenesis. Angiogenesis or establishing a blood supply for growth is essential to ectopic lesions and Notch signaling plays a major role in this process in other contexts. Korbel et al found that the γ-secretase inhibitor DAPT increased angiogenic sprouts in lesions[53]. They postulated this increase could lead to a reduction in lesion size and decreased function based on similar results in tumor studies[53]. Significant differences in NOTCH1 expression and activation have been identified in ectopic (Ec) and EuE, including increased N1ICD expression that is associated with increased proliferation measured by Ki-67 expression, ER expression, and decreased PR expression in EcE[54]. Given these data, the authors suggest that increased Notch1 signaling in Ec may contribute to P4 resistance since inhibition of Notch signaling with DAPT helped to sensitize immortalized uterine fibroblasts, HESCs, to P4[54]. Additionally, Notch signaling is linked with oxidative stress and severity of disease in endometriosis. A study of peritoneal fluid and stromal cells isolated from eutopic and ectopic endometrium from women with deep infiltrating endometriosis showed higher ADAM17 expression and activity, higher Notch signaling activation, and that these changes are associated with increased oxidative stress and lesion fibrosis[55]. Notch signaling is also activated by immune modulation in many other contexts, as well as in EcE. In our study of ectopic tissue, we identified increased NOTCH1 expression in humans and in our baboon model of endometriosis[56]. In addition to these alterations in expression, we elucidated a potential mechanism of Notch1 signaling regulation in the epithelium of lesions where IL-6 regulates P38/MAPK signaling to induce NOTCH1 promoter occupancy of E2A/HEB[56]. Notch signaling is altered in Ec and EuE of women with endometriosis providing potential therapeutic targets for future studies to identify to combat this pathology (Figure 3).
Adenomyosis
Adenomyosis is a disease characterized by endometrial tissue found within the myometrium resulting in dysmenorrhea, dyspareunia, abnormal uterine bleeding, and infertility[57]. A histological study performed on endometria from fertile women and women with adenomyosis revealed increased NOTCH1 expression in ectopic endometria of women with adenomyosis[58]. Expression of an inhibitory regulator of Notch signaling, NUMB, is decreased in adenomyosis lesions within the myometrium[58]. Decreased NUMB expression may explain the increased NOTCH1 expression noted in adenomyosis lesions. Functional analyses revealed that adenomyosis lesions exhibit increased Slug and Snail, known markers of epithelial to mesenchymal transition (EMT), transcriptional expression that is mediated by NOTCH1 signaling activation thereby inducing EMT that contributes to the pathogenesis of adenomyosis[58]. The etiology and pathophysiology of adenomyosis remain significant areas of study, but these results suggest that the Notch signaling pathway may be an important contributor to the disease warranting future investigation of the role of Notch signaling in adenomyosis.
Pre-eclampsia
Pre-eclampsia is a pregnancy disorder that is characterized by hypertension and proteinuria in the mother. This disease affects 3–5% of pregnancies and can result in significant health risks for both mother and fetus including death[59]. NOTCH1 and NOTCH4 are decreased in pre-eclamptic placentas compared to normal control placentas suggesting that defective Notch signaling may contribute to the etiology of this disease[60]. A study performed in vitro indicates that inhibition of Notch signaling resulted in reduced trophoblast invasion suggesting a role for Notch signaling in placental invasion[61]. In addition, the authors also noted that NOTCH2 deletion resulted in placental development defects, which resulted in offspring lethality[61]. The study also suggests that decreased JAG1 expression combined with the role of Notch signaling in vascular development could contribute to pre-eclampsia[61].
Infertility
Several studies provide a role for Notch signaling in infertility and uterine repair. We utilized a murine model to constitutively activate Notch1 signaling through overexpression of the Notch1 intracellular domain (Pgrcre/+ Rosa26N1ICD/+)[32]. This constitutive activation of Notch1 signaling resulted in a glandless phenotype that resulted in infertility suggesting that Notch1 signaling is critical for glandular development in mice[32]. In addition to being infertile, these mice fail to respond to decidualization stimuli and display decreased P4 responsiveness highlighting the importance of Notch signaling in regulating and responding to hormonal signals[32]. Further investigation uncovered that N1ICD overexpression results in hypermethylation of the progesterone receptor through activation of a PU.1/Dnmt3b complex and this reduction allowed for an increase in estrogen signaling compromising decidualization and implantation[32]. We also generated Pgrcre/+ RbpjFl/Fl mice to conditionally delete all Notch signaling in the mouse uterus[62]. These mice display a recurrent pregnancy loss (RPL) phenotype where mice give birth to a normal sized first litter, but subsequent litters decrease in size, and these mice show a reduced decidualization response compared to controls[62]. We noted postpartum nodules following parturition in these mice that did not regress normally and an abnormal inflammatory environment that compromised implantation in future pregnancies[62]. Furthermore, in this study we found that women with recurrent pregnancy loss also exhibited reduced RBPJ expression suggesting that women exhibiting recurrent pregnancy loss may have dysregulated uterine repair mechanisms contributing to their pathology[62]. In addition, endometria from infertile women exhibit decreased NOTCH1 and DLL1 expression and increased NUMB expression by histological analysis[52]. These results indicate that Notch signaling as well as the Notch transcriptional effector, Rbpj may significantly contribute to infertility.
Endometrial Cancer
Endometrial cancer encompasses two subtypes of endometrial carcinoma: Type I endometroid endometrial cancer (EEC) and Type II serous endometrial cancer (SEC). EEC is the more common type of endometrial cancer where the endometrium is hyperplastic, and tumors are ER and PR positive. In SEC, the endometrium is atrophic and tumors are ER independent[63]. Alterations in NOTCH receptor, ligand, and target gene expression are associated with the endometrium of endometrial carcinoma patients. A NOTCH4 decrease was originally identified in endometrial cancer cells and later validated by immunohistochemical analysis in endometrial carcinoma tissue [22, 64]. Conversely, NOTCH1 expression is increased in endometrial cancer and is associated with a poor prognosis[63]. Mixed data also indicate both increases and decreases in JAG1, JAG2, and NOTCH3, while data for other Notch receptor and ligand expression is currently lacking[63]. Aberrations in Notch signaling component expression are present in both non-malignant and malignant gynecologic disorders, suggesting diverse roles for this pathway in reproductive tract abnormalities.
Concluding Remarks and Future Perspectives
Regulation of Notch signaling is an underexplored area of research in the context of reproduction. Due to the importance of this signaling pathway in reproductive tract development, function, and disease, it warrants increased investigation. Current areas of investigation include hormonal regulation of Notch signaling by hCG, E2, and P4, but these mechanisms and interactions are not well understood. It is possible that these hormones may be regulating Notch signaling at the level of ligand presentation, receptor activation, cleavage enzyme activation, or by interacting with the Notch transcriptional activator RBPJ. Other evidence also suggests that Notch may interact with epigenetic modifiers, such as members of the SWI/SNF family, to alter chromatin accessibility, but this has not been thoroughly investigated in the context of reproductive physiology[32, 65]. In addition, dysregulation of Notch signaling has been shown to play a role in developmental defects, reproductive diseases, and cancer. The Notch signaling pathway may be an integral pathway in many different disease etiologies, especially in pathologies of the reproductive tract such as endometriosis, PCOS, pre-eclampsia, adenomyosis, and infertility. Targeting Notch signaling could also provide novel and beneficial therapeutics to advance patient treatment for reproductive pathologies and infertility. Therefore, there are multiple avenues open for further investigation and evidence suggests that Notch signaling, and regulation may differ within the different organs of the reproductive tract.
Outstanding Questions.
What are the upstream regulators of Notch signaling in the male and female reproductive tracts? Are these modifiers hormonal, epigenetic or post-translational in nature?
What roles does Notch signaling play during the process of embryo implantation and how can these mechanisms be utilized to combat implantation failure resulting in infertility?
What are the functional roles of Notch signaling in pathologies associated with gynecological diseases and how can these mechanisms be exploited to identify potential treatment options for disease?
Highlights.
The Notch signaling pathway is an important mediator of cell fate controlling cell proliferation, differentiation, and cell death.
Notch signaling contributes to the reproductive function of the male and female reproductive organs.
Notch signaling is hormonally regulated.
The Notch signaling pathway is implicated in gynecological pathologies like polycystic ovary syndrome, adenomyosis, endometriosis, pre-eclampsia, recurrent pregnancy loss and infertility, and endometrial cancer.
Acknowledgements
The authors would like to thank previous laboratory members Yalda Afshar M.D. Ph.D., Ren-Wei Su Ph.D., and Michael Strug D.O. Ph.D., whose diligent work has greatly contributed to our understanding of Notch signaling in the endometrial environment. Research reported in this review was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award numbers R01HD042280 to A.T.F and T32HD087166 to A.T.F and G.E.M. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kopan R (2012) Notch signaling. Cold Spring Harb Perspect Biol 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Souza B, et al. (2010) Canonical and Non-Canonical Notch Ligands. In Notch Signaling, pp. 73–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aster JC, et al. (2017) The Varied Roles of Notch in Cancer. Annu Rev Pathol 12, 245–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntyre B, et al. (2020) Overview of Basic Mechanisms of Notch Signaling in Development and Disease. Adv Exp Med Biol 1227, 9–27 [DOI] [PubMed] [Google Scholar]
- 5.Su RW and Fazleabas AT (2015) Implantation and Establishment of Pregnancy in Human and Nonhuman Primates. Adv Anat Embryol Cell Biol 216, 189–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murta D, et al. (2015) Dynamics of Notch signalling in the mouse oviduct and uterus during the oestrous cycle. Reprod Fertil Dev [DOI] [PubMed] [Google Scholar]
- 7.Afshar Y, et al. (2012) Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology 153, 2884–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fazleabas AT, et al. (1999) Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci U S A 96, 2543–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strug MR, et al. (2016) Intrauterine human chorionic gonadotropin infusion in oocyte donors promotes endometrial synchrony and induction of early decidual markers for stromal survival: a randomized clinical trial. Hum Reprod 31, 1552–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caliceti C, et al. (2013) 17beta-estradiol enhances signalling mediated by VEGF-A-delta-like ligand 4-notch1 axis in human endothelial cells. PLoS One 8, e71440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzo P, et al. (2009) Targeting Notch signaling cross-talk with estrogen receptor and ErbB-2 in breast cancer. Adv Enzyme Regul 49, 134–141 [DOI] [PubMed] [Google Scholar]
- 12.Hao L, et al. (2010) Notch-1 activates estrogen receptor-alpha-dependent transcription via IKKalpha in breast cancer cells. Oncogene 29, 201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Y, et al. (2012) Nuclear estrogen receptor-mediated Notch signaling and GPR30-mediated PI3K/AKT signaling in the regulation of endometrial cancer cell proliferation. Oncol Rep 27, 504–510 [DOI] [PubMed] [Google Scholar]
- 14.Chang YH, et al. (2019) Estradiol and Progesterone Induced Differentiation and Increased Stemness Gene Expression of Human Fallopian Tube Epithelial Cells. J Cancer 10, 3028–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi T, et al. (2001) Requirement of Notch 1 and its ligand jagged 2 expressions for spermatogenesis in rat and human testes. J Androl 22, 999–1011 [DOI] [PubMed] [Google Scholar]
- 16.Lupien M, et al. (2006) Expression of constitutively active Notch1 in male genital tracts results in ectopic growth and blockage of efferent ducts, epididymal hyperplasia and sterility. Dev Biol 300, 497–511 [DOI] [PubMed] [Google Scholar]
- 17.Murta D, et al. (2014) In vivo notch signaling blockade induces abnormal spermatogenesis in the mouse. PLoS One 9, e113365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rochette MJ and Murphy MP (2002) Gamma-secretase: substrates and inhibitors. Mol Neurobiol 26, 81–95 [DOI] [PubMed] [Google Scholar]
- 19.Hitzenberger M, et al. (2020) The dynamics of gamma-secretase and its substrates. Semin Cell Dev Biol 105, 86–101 [DOI] [PubMed] [Google Scholar]
- 20.Murta D, et al. (2013) Dynamics of Notch pathway expression during mouse testis postnatal development and along the spermatogenic cycle. PLoS One 8, e72767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murta D, et al. (2016) Notch signaling in the epididymal epithelium regulates sperm motility and is transferred at a distance within epididymosomes. Andrology 4, 314–327 [DOI] [PubMed] [Google Scholar]
- 22.Cobellis L, et al. (2008) The pattern of expression of Notch protein members in normal and pathological endometrium. J Anat 213, 464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones RE and Lopez KH (2014) Human reproductive biology. (Fourth edition. edn), pp. xi, 381 pages, Elsevier/AP, Academic Press is an imprint of Elsevier, [Google Scholar]
- 24.Vanorny DA and Mayo KE (2017) The role of Notch signaling in the mammalian ovary. Reproduction 153, R187–R204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanorny DA, et al. (2014) Notch signaling regulates ovarian follicle formation and coordinates follicular growth. Mol Endocrinol 28, 499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasasya RD and Mayo KE (2018) Notch Signaling Regulates Differentiation and Steroidogenesis in Female Mouse Ovarian Granulosa Cells. Endocrinology 159, 184–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terauchi KJ, et al. (2016) Role of Notch signaling in granulosa cell proliferation and polyovular follicle induction during folliculogenesis in mouse ovary. Cell Tissue Res 365, 197–208 [DOI] [PubMed] [Google Scholar]
- 28.Hubbard N, et al. (2019) Activation of Notch Signaling by Oocytes and Jag1 in Mouse Ovarian Granulosa Cells. Endocrinology 160, 2863–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afshar Y, et al. (2012) Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB J 26, 282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su RW, et al. (2015) Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J Clin Endocrinol Metab 100, E433–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, et al. (2021) Notch1 is crucial for decidualization and maintaining the first pregnancy in the mouse. Biol Reprod 104, 539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su RW, et al. (2016) Aberrant activation of canonical Notch1 signaling in the mouse uterus decreases progesterone receptor by hypermethylation and leads to infertility. Proc Natl Acad Sci U S A 113, 2300–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strug MR, et al. (2018) The Notch Family Transcription Factor, RBPJkappa, Modulates Glucose Transporter and Ovarian Steroid Hormone Receptor Expression During Decidualization. Reprod Sci, 1933719118799209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otti GR, et al. (2014) Notch2 controls prolactin and insulin-like growth factor binding protein-1 expression in decidualizing human stromal cells of early pregnancy. PLoS One 9, e112723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, et al. (2019) poFUT1 promotes endometrial decidualization by enhancing the O-fucosylation of Notch1. EBioMedicine 44, 563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shawber CJ, et al. (2015) Vascular Notch proteins and Notch signaling in the peri-implantation mouse uterus. Vasc Cell 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchetto NM, et al. (2020) Endothelial Jagged1 Antagonizes Dll4/Notch Signaling in Decidual Angiogenesis during Early Mouse Pregnancy. Int J Mol Sci 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuman C, et al. (2014) Fetal-maternal communication: the role of Notch signalling in embryo implantation. Reproduction 147, R75–86 [DOI] [PubMed] [Google Scholar]
- 39.Cuman C, et al. (2013) Preimplantation human blastocysts release factors that differentially alter human endometrial epithelial cell adhesion and gene expression relative to IVF success. Hum Reprod 28, 1161–1171 [DOI] [PubMed] [Google Scholar]
- 40.Zhang S, et al. (2014) Uterine Rbpj is required for embryonic-uterine orientation and decidual remodeling via Notch pathway-independent and -dependent mechanisms. Cell Res 24, 925–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levin HI, et al. (2017) Dynamic maternal and fetal Notch activity and expression in placentation. Placenta 55, 5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haider S, et al. (2017) Notch signalling in placental development and gestational diseases. Placenta 56, 65–72 [DOI] [PubMed] [Google Scholar]
- 43.Lu J, et al. (2019) Spatiotemporal coordination of trophoblast and allantoic Rbpj signaling directs normal placental morphogenesis. Cell Death Dis 10, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escobar-Morreale HF (2018) Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 14, 270–284 [DOI] [PubMed] [Google Scholar]
- 45.Amjadi F, et al. (2019) Comparative evaluation of NOTCH signaling molecules in the endometrium of women with various gynecological diseases during the window of implantation. Iran J Basic Med Sci 22, 426–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang D, et al. (2020) Identification of Potential Biomarkers of Polycystic Ovary Syndrome via Integrated Bioinformatics Analysis. Reprod Sci [DOI] [PubMed] [Google Scholar]
- 47.Giudice LC and Kao LC (2004) Endometriosis. Lancet 364, 1789–1799 [DOI] [PubMed] [Google Scholar]
- 48.Buzzio OL, et al. (2006) FOXO1A differentially regulates genes of decidualization. Endocrinology 147, 3870–3876 [DOI] [PubMed] [Google Scholar]
- 49.Kim JJ, et al. (2005) Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol Reprod 73, 833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JJ and Fazleabas AT (2004) Uterine receptivity and implantation: the regulation and action of insulin-like growth factor binding protein-1 (IGFBP-1), HOXA10 and forkhead transcription factor-1 (FOXO-1) in the baboon endometrium. Reprod Biol Endocrinol 2, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park Y, et al. (2016) Cis-Regulatory Evolution of Forkhead Box O1 (FOXO1), a Terminal Selector Gene for Decidual Stromal Cell Identity. Mol Biol Evol 33, 3161–3169 [DOI] [PubMed] [Google Scholar]
- 52.Van Sinderen M, et al. (2014) Localisation of the Notch family in the human endometrium of fertile and infertile women. J Mol Histol 45, 697–706 [DOI] [PubMed] [Google Scholar]
- 53.Korbel C, et al. (2018) Notch signaling controls sprouting angiogenesis of endometriotic lesions. Angiogenesis 21, 37–46 [DOI] [PubMed] [Google Scholar]
- 54.Brown DM, et al. (2018) Notch-1 Signaling Activation and Progesterone Receptor Expression in Ectopic Lesions of Women With Endometriosis. J Endocr Soc 2, 765–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez-Foruria I, et al. (2017) Dysregulation of the ADAM17/Notch signalling pathways in endometriosis: from oxidative stress to fibrosis. Mol Hum Reprod 23, 488–499 [DOI] [PubMed] [Google Scholar]
- 56.Song Y, et al. (2020) Interleukin-6 (IL-6) Activates the NOTCH1 Signaling Pathway Through E-Proteins in Endometriotic Lesions. J Clin Endocrinol Metab 105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taran FA, et al. (2013) Adenomyosis: Epidemiology, Risk Factors, Clinical Phenotype and Surgical and Interventional Alternatives to Hysterectomy. Geburtshilfe Frauenheilkd 73, 924–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qi S, et al. (2015) Aberrant expression of Notch1/numb/snail signaling, an epithelial mesenchymal transition related pathway, in adenomyosis. Reprod Biol Endocrinol 13, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steegers EA, et al. (2010) Pre-eclampsia. Lancet 376, 631–644 [DOI] [PubMed] [Google Scholar]
- 60.Cobellis L, et al. (2007) Distribution of Notch protein members in normal and preeclampsia-complicated placentas. Cell Tissue Res 330, 527–534 [DOI] [PubMed] [Google Scholar]
- 61.Hunkapiller NM, et al. (2011) A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development 138, 2987–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strug MR, et al. (2018) RBPJ mediates uterine repair in the mouse and is reduced in women with recurrent pregnancy loss. FASEB J 32, 2452–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jonusiene V and Sasnauskiene A (2021) Notch and Endometrial Cancer. Adv Exp Med Biol 1287, 47–57 [DOI] [PubMed] [Google Scholar]
- 64.Suzuki T, et al. (2000) Imbalanced expression of TAN-1 and human Notch4 in endometrial cancers. Int J Oncol 17, 1131–1139 [DOI] [PubMed] [Google Scholar]
- 65.Pillidge Z and Bray SJ (2019) SWI/SNF chromatin remodeling controls Notch-responsive enhancer accessibility. EMBO Rep 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kopan R and Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D’Souza B, et al. (2010) Canonical and non-canonical Notch ligands. Curr Top Dev Biol 92, 73–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miele L (2006) Notch signaling. Clin Cancer Res 12, 1074–1079 [DOI] [PubMed] [Google Scholar]
- 69.Moloney DJ, et al. (2000) Fringe is a glycosyltransferase that modifies Notch. Nature 406, 369–375 [DOI] [PubMed] [Google Scholar]
- 70.Carrieri FA, et al. (2019) CDK1 and CDK2 regulate NICD1 turnover and the periodicity of the segmentation clock. EMBO reports [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin S, et al. (2019) Deubiquitylation and stabilization of Notch1 intracellular domain by ubiquitin-specific protease 8 enhance tumorigenesis in breast cancer. Cell Death Differ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maier J, et al. (2019) Luminescent and fluorescent triple reporter plasmid constructs for Wnt, Hedgehog and Notch pathway. PLoS One 14, e0226570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ochoa-Bernal MA and Fazleabas AT (2020) Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int J Mol Sci 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Croy BA, et al. (2014) The guide to investigation of mouse pregnancy. pp. xx, 808 pages, Elsevier/Academic Press, [Google Scholar]
- 75.Makieva S, et al. (2018) Inside the Endometrial Cell Signaling Subway: Mind the Gap(s). Int J Mol Sci 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dunn CL, et al. (2003) Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online 7, 151–161 [DOI] [PubMed] [Google Scholar]
- 77.Cha J, et al. (2012) Mechanisms of implantation: strategies for successful pregnancy. Nat Med 18, 1754–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hemberger M, et al. (2020) Mechanisms of early placental development in mouse and humans. Nat Rev Genet 21, 27–43 [DOI] [PubMed] [Google Scholar]


