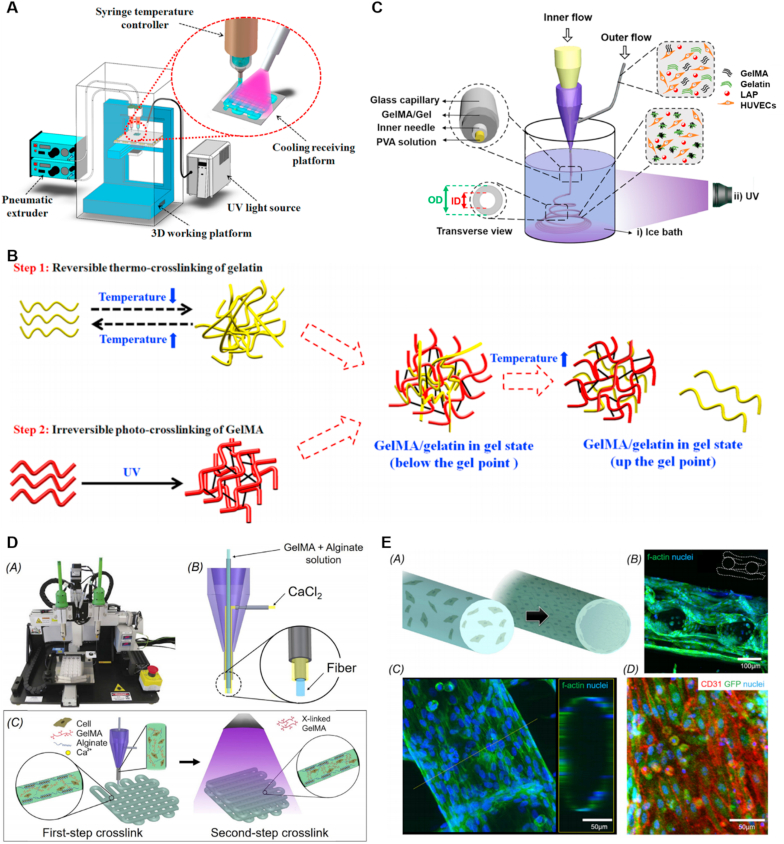
Fig. 4.
(A) Schematic of the 3D bioprinting system: the temperature controller on the syringe regulated the viscosity of GelMA/gelatin bioinks; the cooling system under the receiving platform temporarily thermo-cross-linked gelatin in the bioinks; and the UV light permanently photo-crosslinked GelMA in the bioinks. (B) Two-step cross-linking of GelMA/gelatin bioinks. Reprinted with permission from Ref. [132]. Copyright 2018, ACS. (C) Schematic illustrating the setup for generating hollow GelMA/Gel microfibers by microfluidic coaxial biofabrication for cannular tissue engineering. Reprinted with permission from Ref. [50]. Copyright 2019, ACS. (D) Photograph of an Organovo bioprinter. Schematic of the coaxial needle where the bioink is delivered from the core and the ionic crosslinking CaCl2 solution is sheathed on the side. Schematic diagrams showing the two-step crosslinking process, where the alginate component is first physically crosslinked by the CaCl2 followed by chemical crosslinking of the GelMA component using UV illumination. Reprinted with permission from Ref. [49]. Copyright 2016, Elsevier.