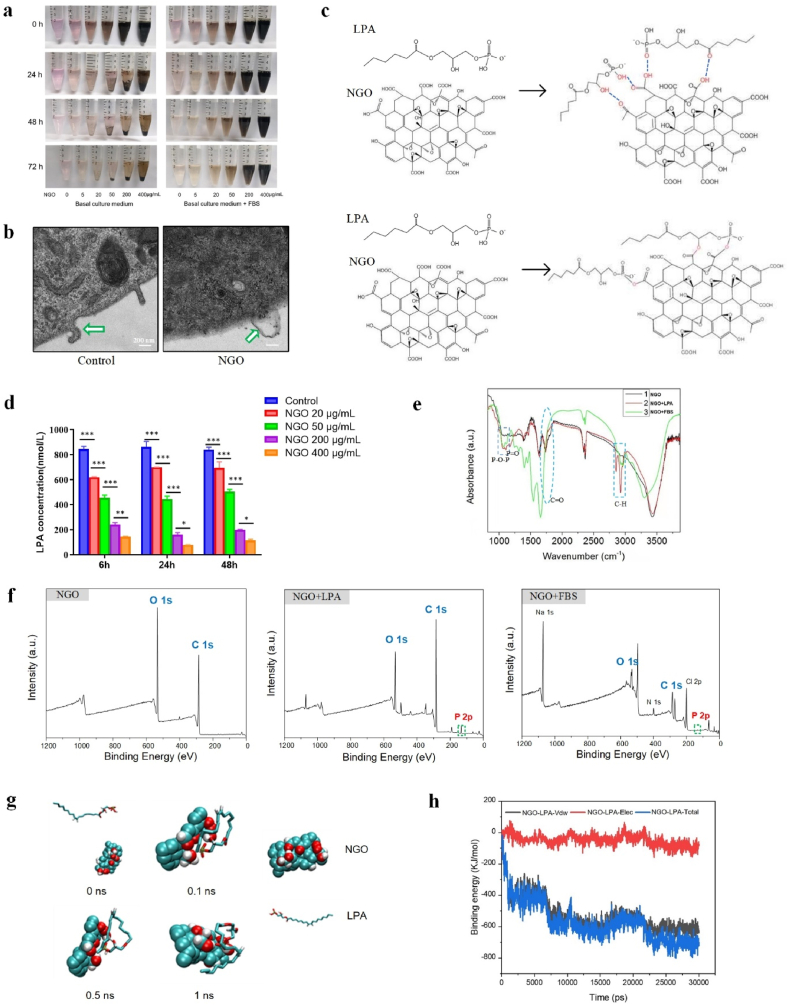
Fig. 4.
Characterization of NGO-adsorbed LPA. (a) Images of NGO dispersed in basal culture medium and complete culture medium (basal culture medium + FBS) for 0, 24, 48 and 72 h. (b) TEM images of HUVECs incubated with 5 μg/mL NGO for 24 h at 37 °C; the interface between NGO and the cell membrane is indicated by the white arrow. Scale bars, 200 nm. (c) Schematic illustrations showing the mechanism of NGO loading with LPA. Hydrogen bonds can be formed between NGO and LPA, or esterification reactions may occur. (d) The concentration of LPA in solution after incubation with 20, 50, 200 or 400 μg/mL NGO for 6, 24 or 48 h at 37 °C was measured with an ELISA kit. Error bars are standard deviation across three repetitive experiments. *p < 0.01, **p < 0.001, ***p < 0.0001. (e) FTIR analysis demonstrating typical vibrations corresponding to the chemical structure of LPA; the C O vibration peak in NGO (5 μg/mL) was partially eliminated after incubation in the presence of LPA or FBS for 24 h at 37 °C. (f) XPS analysis showing that the content of P increased in NGO (5 μg/mL) after incubation in the presence of LPA or FBS for 24 h at 37 °C. (g) The self-assembly of NGO and LPA. (h) The binding energy of LPA-NGO during 30 ns simulations. (*Vdw represents van der Waals forces, Elec represents electrostatic interactions and Total represents the total binding energy).