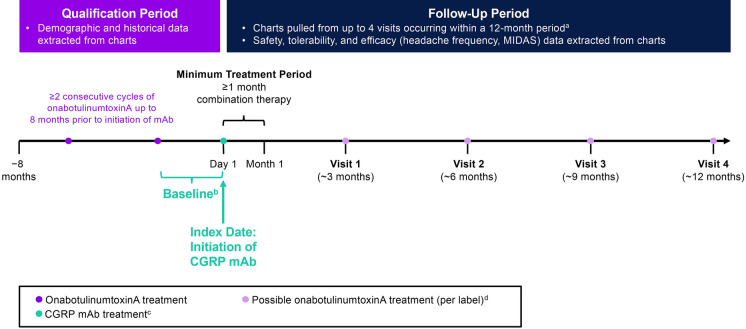
Fig. 1.
Study design. aNot all patients had four visits or 12 months of data. bBaseline assessments for outcome measures (e.g., headache day frequency, headache intensity, disability) were collected from the visit at which the CGRP mAb was prescribed and reflect patient assessments during approximately 1–3 months prior to initiation of the CGRP mAb. cCGRP mAbs were self-administered by subcutaneous injection. Per label, erenumab and galcanezumab are administered once monthly, and fremanezumab is administered once every 3 months. dOnabotulinumtoxinA treatment is not always administered per label. CGRP calcitonin gene–related peptide, mAb monoclonal antibody, MIDAS Migraine Disability Assessment