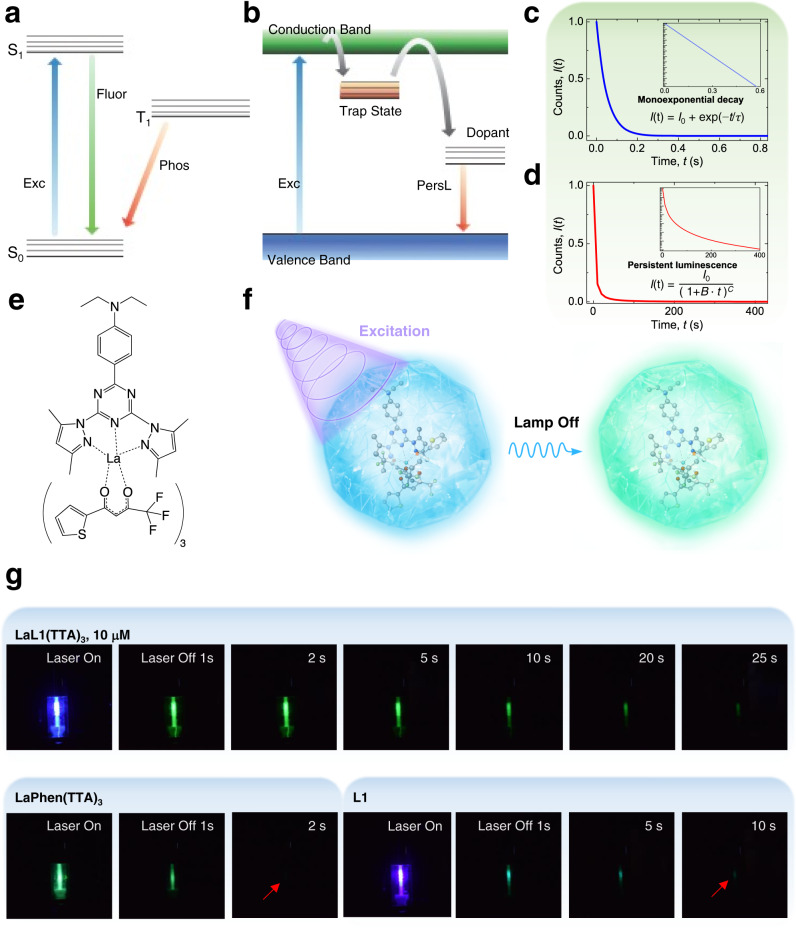
Fig. 1. Differences between phosphorescence and persistent luminescence and performance of LaL1(TTA)3.
The schematic Jablonski diagram of (a) fluorescence (Fluor) and phosphorescence (Phos) of an organo-lanthanide complex upon excitation (Exc) with the absence of energy transfer to the trivalent lanthanide ion. Phosphorescence usually only occurs under cryogenic condition due to the high nonradiative rate T1 → S0 at room temperature. (b) Persistent luminescence (PersL) may occur when trapped electrons (or holes) transfer to the lanthanide dopant ions through thermal release via the conduction band or tunneling. (c) The ideal exponential luminescence decay of phosphorescence with lifetime 0.05 s. (d) Ideal hyperbolic decay of persistent luminescence with rate constant B = 0.25 s−1. The insets of (c) and (d) display ordinates on logarithmic scales. (e) The chemical structure of LaL1(TTA)3. (f) The schematic illustration with a photograph of the long-lived afterglow phosphorescence of LaL1(TTA)3 after 355 nm excitation. (g) The long-lasting afterglow of LaL1(TTA)3 at 10 μM concentration in toluene is observable for up to 30 s after Nd3+:YAG laser irradiation at 77 K. The afterglow of LaPhen(TTA)3 (diminished after 2 to 3 s) and of L1 (just above 5 s) are shown for comparison. (L1 = 2-(N,N-diethylanilin-4-yl)−4,6-bis(3,5-dimethylpyrazol-1-yl)-1,3,5-triazine (dbpt); TTA = thenoyltrifluoroacetonate; Phen = 1, 10-phenanthroline).