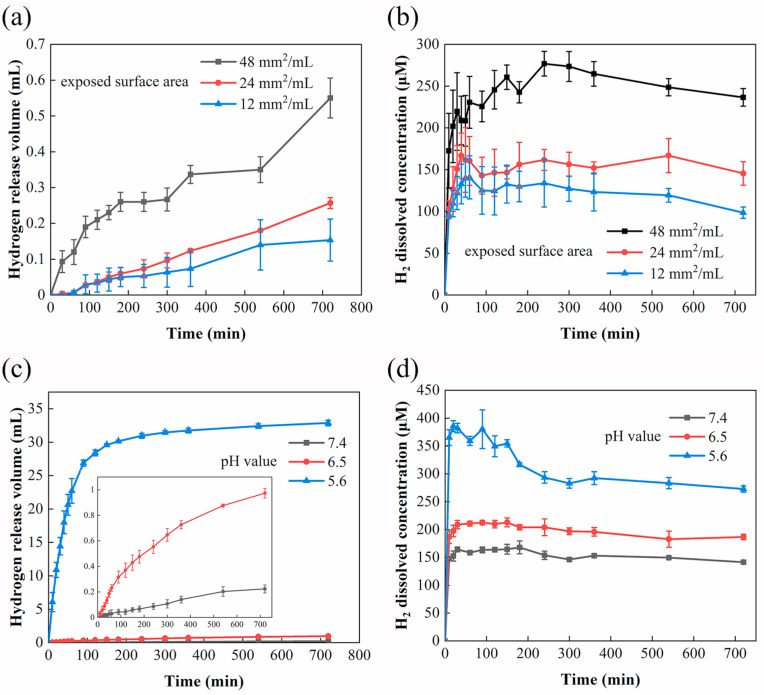
Fig. 1.
Controlled release kinetics of hydrogen achieved by changing the surface area of Mg or the pH value of PBS. (a) The volume of released H2 and (b) the concentration of dissolved H2 in PBS solution increased with the exposed surface area (mm2 per mL PBS) of Mg plates (pH = 7.4). (c) The volume of released H2 and (d) the concentration of dissolved H2 in PBS increased as the pH value decreases.