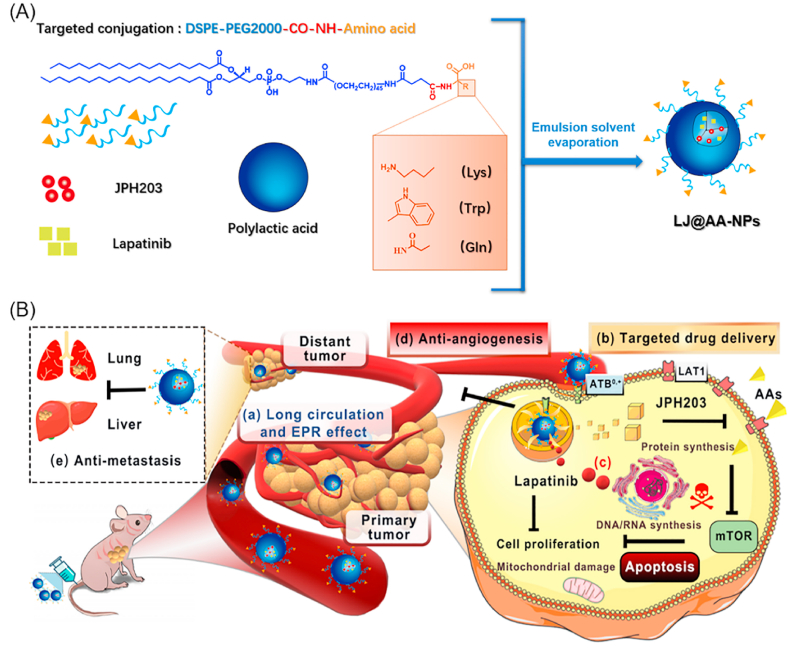
Fig. 1.
Schematic illustration for the design of ATB0,+-targeted nanoparticles and its application for cancer therapy. (A) The design and construction of lapatinib/JPH203-coloaded amino acid-conjugated nanoparticles (LJ@AA-NPs). (B) The anticancer mechanism of LJ@AA-NPs. (a) Upon injection, LJ@AA-NPs exhibit prolonged half-life in circulation and accumulate in tumors. (b) The conjugated amino acid interacts with ATB0,+ on tumor-cell surface and mediates the enhanced uptake of NPs; ATB0,+ protein gets down-regulated in the process. (c) After endocytosed into cells, lapatinib and JPH203 are released inside the cells; JPH203 blocks LAT1-mediated amino acid delivery, which inactivates mTOR function. The decreased function of ATB0,+ due to its down-regulation also contributes to amino acid starvation in cancer cells. Lapatinib exerts anticancer effect by itself by blocking the growth-promoting signaling of EGFR/HER1 and HER2. LJ@AA-NPs also (d) prevent angiogenesis and (e) exert anti-metastatic effects.