Abstract
Purpose of Review
Three COVID-19 vaccines obtained emergency authorization from the Food and Drug Administration (FDA) and are widely used in the USA. Unfortunately, there is a paucity of evidence on the safety and efficacy of these vaccines in patients with autoimmune inflammatory rheumatic diseases (AIIRD), as these patients were excluded from all phases of vaccine development. Here we reviewed current data on COVID-19 vaccination in patients with AIIRD, with emphasis on systemic lupus erythematosus (SLE), and provided a comprehensive update on the benefits and risks of vaccination.
Recent Findings
Patients with SLE have worse immune responses following SARS-CoV-2 vaccination than healthy controls. The efficacy of the COVID-19 vaccines seems to be further reduced by immunosuppressive medications, such as glucocorticoids (GC), methotrexate (MTX), mycophenolate/mycophenolic acid (MMF), and rituximab (RTX). However, these data do not substantiate that AIIRD patients are at greater risk of disease flares or have a higher incidence of side effects following vaccination. There is no significant safety concern for the use of COVID-19 vaccines in patients with AIIRD.
Summary
The benefits of vaccination far outweigh the risks in patients with AIIRD, including SLE. More data are needed to determine the necessity of a booster vaccine dose and appropriate adjustment of immunosuppressants around the administration of vaccine.
Keywords: COVID-19 vaccines, SARS-CoV-2, Systemic lupus erythematosus, Rheumatic diseases, Immunosuppressive agents
Introduction
Since the start of the COVID-19 pandemic in March 2020, scientists and researchers have made enormous efforts to develop safe and effective vaccines against SARS-CoV-2 [1]. Three vaccines, two mRNA vaccines (Pfizer/BioNTech BNT162b2 and Moderna mRNA-1273), and one adenovirus-based vaccine (Janssen Ad26.COV2.S) have completed phase III trials and received emergency use authorization (EUA) in the USA [2–4]. As of August 23, 2021, the Pfizer/BioNTech BNT162b2 obtained full approval for use in individuals ages 16 and older to prevent COVID-19 disease [5]. Another adenovirus-based vaccine (AstraZeneca ChAdOx1 nCoV-19) has been widely used in Europe while still awaiting EUA from the FDA to enter the USA [6]. Patients with SLE were reported to be at higher risk of SARS-CoV-2 infection and worse outcomes from COVID-19, possibly due to their intrinsic immune dysfunction, demographics, disease activity, medications, associated organ damage, and comorbidities [7]. Additionally, the immunomodulators and immunosuppressants commonly used to treat SLE have been associated with an increased risk of death from COVID-19 [8]. Therefore, patients with SLE are among the most vulnerable population in terms of SARS-CoV-2 infection and, as such, have been among the first to receive the vaccines [9]. However, a large proportion of patients with AIIRD express vaccine refusal or hesitancy due to concerns for adverse events and the lack of long-term research on their safety [10]. Phase III trials of all the above four vaccines excluded patients treated with immunosuppressants or immune-modifying drugs within six months of enrollment, thus resulting in a visual absence of safety and efficacy data in patients with autoimmune inflammatory rheumatic diseases (AIIRD), including SLE [2–4, 6]. Furthermore, there is no evidence as to whether specific treatments should be delayed or paused around the administration of COVID-19 vaccines or whether patients with AIIRD can benefit from a booster dose of vaccination [11]. Hopefully, the ongoing ACV01 study, a randomized, multi-site, adaptive, open-label clinical trial investigating booster effects with autoimmune treatments in patients with poor response to initial COVID-19 vaccine, will provide further evidence on this topic [12].
This publication reviews the published data on SARS-CoV-2 vaccination in SLE patients to assist rheumatologists in organizing informed discussions with their lupus patients. A search of published and preprint databases in all languages was performed. The eligibility criteria were as follows: (1) described at least one relevant measurement of efficacy and/or safety profile regarding SARS-CoV-2 vaccine; (2) included patients with AIIRD that contained at least 1 patient with SLE; (3) was published after January 1, 2020; and (4) was original research. Three independent reviewers (WT, LK, and ADA) screened articles and extracted data. A meta-analysis of effect sizes using fixed- or random-effects model was performed on the topics of special interest when possible. All analyses were conducted using RevMan 5.4 software.
Efficacy of COVID-19 Vaccines in Patients with Systemic Lupus
Erythematosus
SLE is characterized by profound dysregulation of the immune system. Both qualitative and quantitative lymphocyte defects are prevalent in SLE as a result of both the underlying disease activity and treatment with immunosuppressants [9]. SLE patients have been reported to have poor immune responses to vaccines against several pathogens [13].
A total of 11 studies evaluated COVID-19 vaccine efficacy in AIIRD patients that included SLE. Two types of responses were considered, anti-spike IgG and/or IgM seroconversion rates and neutralizing antibody activity against SARS-CoV-2. These data are summarized in Table 1. Izmirly et al. showed that fully vaccinated SLE patients (n=90) produced significantly lower IgG antibodies against SARS-CoV-2 spike receptor binding domain (RBD) than healthy controls (HCs) (n=20) [14]. Boekel et al. reported that among participants without previous SARS-CoV-2 infection, seroconversion rates after first COVID-19 vaccination in patients with AIIRDs (n=432) were lower than HCs (n=210), an effect that was mainly driven by those treated with MTX or B cell-depletion therapies; however, differences in seroconversion rates were similar across autoimmune disease types examined [15]. Similarly, Ammitzbøll et al. observed a higher seroconversion rate following BNT162b2 vaccine in SLE (n=73) than RA (n=64) patients, but the difference was not statistically significant after adjusting for RTX treatment [16]. Decreases in vaccine efficacy have also been observed in B cell lymphoma patients following B cell depletion therapy [17]. Taken together, these studies suggest that immunosuppressive treatments, rather than the underlying autoimmune diseases, might be the main factor limiting the immune responses against SARS-CoV-2 vaccines. Unexpectedly, Simon et al. reported lower antibody responses to SARS-CoV-2 vaccine in untreated AIIRD patients (n=24) than HCs (n=182), and further comparison between conventional synthetic disease-modifying antirheumatic drugs (DMARDs)- and biological/target-synthetic DMARDs-treated patients revealed no differences in antibody responses [18]. Furer et al. found that the seroconversion rates and neutralizing antibody levels following COVID-19 vaccination were significantly lower in patients with AIIRD as compared to HCs and significantly different across different disease diagnoses with the lowest seroconversion observed in patients with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) and idiopathic inflammatory myositis (IIM). Notably, seroconversion rates in SLE patients were also significantly lower as compared to HCs in this study, and treatment with immunomodulatory and immunosuppressive agents was found to be the major risk factor for reduced immunogenicity [19].
Table 1.
Summary of studies on COVID-19 vaccine efficacy in patients with autoimmune inflammatory rheumatic disorders
| Author | Design | Vaccine | Cohort | Diseases | Therapy | Sample Collection | Outcome measure | Results |
|---|---|---|---|---|---|---|---|---|
| Izmirly et al. | Cohort | Pfizer, Moderna, J&J |
90 SLE 20 HC |
SLE 90 (100%) | GC 26 (29%), MTX 8 (14.9%), MMF 21 (23%), RTX 3 (3%), BLyS 10 (11%) | Within 4 months of 1st dose, 2 weeks post 2nd dose of Pfizer/Moderna or 1st dose of J&J | Anti-S IgG, functional neutralization titers, Ag-specific IFN-γ production | Lower* Ab and neutralization titers in SLE vs HC, Ab levels of 26 (29%) SLE patients below the lowest level of HCs; Independent predictors of low responses: any immunosuppressant, normal anti-dsDNA, lower platelets, and normal C3 |
| Furer et al. | Cohort | Pfizer |
686 AIIRD 121 HC |
SLE 101 (14.7%); RA 263 (38.3%); SpA 165 (33.9%) | SLE patients: GC 22 (22%), MTX 8 (8%), BCDT 7 (7%), MMF 17 (17%) All patients: GC 55 (21%), MTX 116 (44%), TNFi (18%), BCDT 87 (13%), MMF 38 (4%) | 2–6 weeks post 2nd dose | Anti-S IgG | All AIIRD vs HC: lower* anti-S IgG seroconversion (86% vs 100%); lower* seroconversion rate in patients on GC (66%), MMF (64%), MTX (84%), and RTX (36%). Lower* seroconversion rate in SLE than HCs (92% vs 100%) |
| Deepak et al. | Cohort | Pfizer, Moderna |
133 IMID 53 HC |
SLE 15 (11.3%); RA 38 (28.6%); SpA 20 (15%); IBD 42 (31.6%) | GC 17 (12.8%), MTX 29 (21.8%), HCQ 30 (22.6%), MMF 9 (6.8%), BCDT 10 (7.5%), | Within 2 weeks prior to 1st dose, 20 days post 2nd dose | Anti-S IgG, functional neutralization titers, circulating S-specific plasmablasts | Reduction in anti-S Ab and neutralization titers vs HCs: IMID 3X; GC 10X; Antimetabolites 2-3X, BCDT 36X; TNFi, antimalarial = minimal |
| Ammitzbøll et al. | Cohort | Pfizer | 134 AIIRD | SLE 61 (45.5%); RA 73 (54.5%) | SLE patients: GC 29 (47.5%), MMF 16 (26.2%), MTX 6 (9.8%), RTX 2 (3.3%) | Prior to the first dose, 7 days after the second dose | Anti-S IgM and IgG | Seroconversion rate: AIIRD 103/134 (77%), SLE 54/61 (88%), RA 49/73 (67%); RTX 4/17 (23%), time since RTX did not influence the Ab levels |
| Ruddy et al. | Cohort | Pfizer, Moderna | 404 AIIRD | SLE 87 (22%); IA 180 (45%); overlap CTDs 84 (21%); Myositis 24 (6%), SS 19 (5%) | GC 117 (29%), MTX 94 (23%), MMF 41 (10%), RTX 19 (5%) | Median of 29 (IQR 28, 32) days after the second dose | Anti-RBD Abs | Seroconversion rate: all patients 378/404 (94%), SLE 78/87 (90%). Lower* seroconversion rate in patients on MMF (73%), RTX (79%), and GC (82%) |
| Geisen et al. | Cohort | Pfizer, Moderna |
26 IMID 42 HC |
SLE 2 (7.7%); RA 8 (30.8%); SpA 5 (19.2%); PsO 4 (15.4%); IBD 3 (11.5%) | GC 7 (26.9%), TNFi 13 (50%), csDMARDs 8 (30.8%) | Prior to 1st dose, days 0 and 7 post 2nd dose | Anti-SARS-CoV-2 IgG, neutralizing antibody; anti-SARS-CoV-2 IgA | Lower anti- SARS-CoV-2 IgA and IgG levels, neutralizing activities in IMID patients than HC. TNFi vs cDMARDs vs anti-IL-17 showed no significant difference between these therapies |
| Simon et al. | Cohort | Pfizer |
84 IMID 182 HC |
SpA 27 (32.1%); RA 25 (29.8%); PsO 8 (9.5%); IBD 8 (9.5%); systemic disorders 16 (19.1%) | Biologics (42.9%); csDMARDs (23.9%); no treatment (28.6%) | >10 days post 2nd dose | Anti-S IgG | Compared to HCs, AIIRD patients had delayed and reduced immune responses to COVID-19 vaccines as reflected by a higher incidence of failure to develop neutralizing antibody activity (9.5% vs 0.5%) and longer time period to reach positive for anti-SARS-CoV-2 antibodies |
| Boekel et al. | Cohort | Pfizer, Moderna, AZ, J&J |
632 IMID 289 HC |
SLE 33 (5%); RA 260 (41%); SpA (142); MS 58 (9%); SS 33 (5%); vasculitis 11 (2%) | GC 109 (17%), MTX 223 (35%), TNFi 141(22%), BCDT 27 (4%), | IMID: 34 days (IQR 31, 38) post 1st dose, and 38 days (IQR 30–61) post 2nd dose; HC: 36 days (IQR 34–41) post 1st dose and 42 days (IQR 35–85) post 2nd dose | Anti-RBD IgG | Post 1st dose - Anti-SARS-CoV-2 IgG IMID (210/432, 49%) vs HCs (154/210, 73%); MTX and BCDT: lower* seroconversion than HC. Post 2nd dose: seroconversion rates similar in all treatment groups, except for patients on BCDTs. S/p COVID-19: post 1st dose, seroconversion rates and IgG titers were similar to HC post 1st dose and patients without previous COVID-19 post 2nd dose |
| Boyarsky et al. | Cohort | Pfizer, Moderna | 123 AIIRD | SLE 24 (20%); IA 34 (28%); CTD 35 (29%); SS 16 (13%); myositis 7 (6%) | MTX 13 (11%), MMF 11 (9%), TNFi 17 (14%), RTX 6 (5%), BLyS 10 (8%) | Median (IQR) of 22 (18–26) days after 1st dose | Anti-RBD Abs | 1st dose only; Patients on MMF or RTX less likely* to develop a detectable Ab response |
| Spiera et al. | chart review | Pfizer, Moderna | 89 AIIRD | SLE 9 (10%); RA 23 (26%); SS 10 (11%); GPA 12 (13%) | GC 17 (19%), MTX 13 (15%), MMF 7 (8%), TNFi 9 (10%), BLyS 2 (2%), RTX 30 (34%) | N/A | Anti-S Abs | SLE patients: 7/9 (78%) seropositive All patients: 68/89 (69%) patients seropositive; of 21 seronegative patients, 20 received RTX, one received BLyS |
| Al-Janabi et al. | Cohort | Pfizer, AZ | 120 IMID | SLE 1 (0.8%); RA 10 (8.3%); PsA (20.8%); PsO (89.2%) | Not applicable | Median 34 days (IQR 23-46) after 1st dose | Anti-S IgG | 1st dose only: 15% no detectable Ab; 41% no detectable anti-S1 IgG. MTX reduced response relative to biologics (adjusted OR = 0.31) |
*Statistically significant; X, fold reduction
Pfizer, Pfizer/BioNTech BNT162b2; Moderna, Moderna mRNA-1273; J&J, Janssen Ad26.COV2.S; AZ, AstraZeneca ChAdOx1 nCoV-19
AIIRD autoimmune inflammatory rheumatic diseases, IMID immune-mediated inflammatory disease, HC healthy control, SLE systemic lupus erythematosus, RA rheumatoid arthritis, SpA spondyloarthritis, PsO psoriasis, PsA psoriatic arthritis, IBD inflammatory bowel disease, SS Sjogren syndrome, GPA granulomatosis with polyangiitis, MS multiple sclerosis, IA inflammatory arthritis, CTD overlap connective tissue disorders, DMARD disease-modifying antirheumatic drug, csDMARDs, conventional synthetic DMARDs, HCQ hydroxychloroquine, GC glucocorticoid, MTX methotrexate, AZA azathioprine, LEF leflunomide, MMF mycophenolate mofetil, RTX rituximab, BCDT B-cell depletion therapy, BLyS belimumab, ABA abatacept, TNFi tumor necrosis factor inhibitor, IL-6i interleukin-6 inhibitor, IL-23i interleukin-23 inhibitor, IL-17i interleukin-17 inhibitor, JAKi Janus kinase inhibitor, IQR interquartile range, OR odds ratio, RBD receptor binding domain, anti-dsDNA anti-double-stranded DNA antibody, S SARS-CoV-2 spike protein
In order to better define the immunogenicity of COVID-19 vaccines in patients with SLE, we review studies containing both SLE and HC groups with granular information on immunogenicity data available for extraction and analysis. Studies without a HC group and/or studies without detailed immunogenicity data in SLE patients were excluded from further analysis.
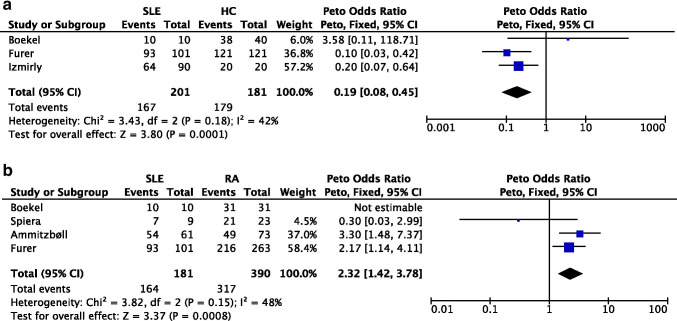
A meta-analysis (Fig 1a) that included three studies of SLE patients and HC (pooled n=382) demonstrated that SLE patients had a lower seroconversion rate following SARS-CoV-2 vaccination than HCs (pooled OR=0.14 [95% CI 0.03, 0.74]). We performed another meta-analysis (Fig 1b) that included four studies of SLE and RA patients (pooled n=571) and found that compared to RA patients, SLE patients had a lower seroconversion rate following vaccination.
Figure 1.
a Meta-analysis of 3 cohorts studies investigating seroconversion rate following SARS-CoV-2 vaccination in SLE patients versus HC. b Meta-analysis of 3 cohort studies investigating seroconversion rate following SARS-CoV-2 vaccination in SLE versus RA patients
Effects of Immunosuppressive Agents on COVID-19 Vaccine Immunogenicity
Emerging data have demonstrated that immunosuppression delays or impairs immune responses to SARS-CoV-2 vaccines in patients with AIIRD, as suggested by previous experience with influenza and pneumococcal vaccines [20]. As shown in Table 1, multiple studies compared vaccine responses among AIIRD patients on different immunosuppressive regimens to define the immunosuppressants responsible for the reduction in these humoral responses. Deepak et al. conducted a cohort study to compare immunogenicity of SARS-CoV-2 mRNA vaccines in AIIRD patients (n=133) and immunocompetent controls (n=53). They identified that the strongest effect on humoral responses was due to B-cell depleting therapies (BCDT) and GC (36- and 10-fold reduction of anti-spike IgG titers), followed by JAKi and antimetabolites including MTX [21]. Another large observational study by Furer et al. identified treatment with GC, RTX, MMF, and abatacept (ABA) as risk factors for the reduced immunogenicity of mRNA BNTb262 among AIIRD patients (n=686) [19]. Similarly, in the SLE cohort study by Izmirly et al., treatment with any non-antimalarial immunosuppressants was independently associated with decreased responses to COVID-19 vaccines in SLE patients (n=90) [14].
Below we reviewed updated evidence on the impacts of several immunosuppressive agents on the serological responses to SARS-CoV-2 vaccines in patients with AIIRD. These immunosuppressants are of particular interest as they are frequently used in the treatment of SLE.
Glucocorticoids
Prednisone dose of ≥10 mg/day was reported to hamper serological responses to pneumococcal vaccination in patients with various immune-mediated inflammatory disorders (IMID) [22]. In SLE patients, high-dose (>20mg/day) prednisone resulted in lower seroconversion after vaccination against influenza A/H1NA [23]. Multiple studies on vaccination against SARS-CoV-2 in AIIRD patients confirmed an association between GC use and dampened antibody responses [14, 19, 21, 24]. Of note, Deepak et al. observed very low anti-SARS-CoV-2 antibody titers and neutralizing activity in some patients taking prednisone < 5mg/day and pointed out that GC had a dose-independent effect on reduction in antibody titers following SARS-CoV-2 vaccination [21].
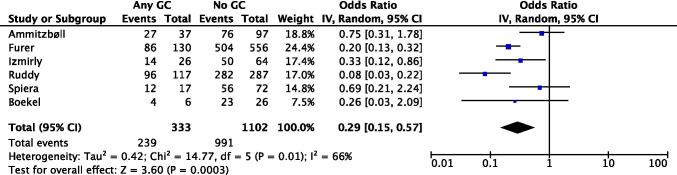
In our meta-analysis (Fig 2) including 6 observational studies (pooled n=1435), GC use was associated with a lower seroconversion rate following SARS-CoV-2 vaccination (pooled OR=0.29 [95% CI 0.15, 0.57]) in patients with AIIRD; we believe this should hold true in SLE. Of concern this meta-analysis included a highly heterogeneous population, with multiple AIIRDs, and incomplete data on non-GC users.
Fig 2.
Meta-analysis of 6 observational studies investigating glucocorticoid use and seroconversion following SARS-CoV-2 vaccination in AIIRD patients
Methotrexate
MTX is a decades-old antimetabolite widely used in rheumatic diseases. A 2013 meta-analysis including a total of 1303 RA patients from 12 studies concluded that MTX decreased the immune responses to pneumococcal and influenza vaccination [25]. Two randomized clinical trials (RCT) by Park et al. further revealed that a temporary discontinuation of MTX following seasonal influenza vaccination, specifically for a duration of 2 weeks, improved its immunogenicity in RA patients [26, 27].
A more recent cohort study by Haberman et al. compared humoral and cellular immune responses to two doses of BNT162b2 mRNA COVID-19 vaccine among individuals with IMID on MTX (n=45), with IMID not on MTX (n=37), and HCs (n=208). Individuals with IMID on MTX demonstrated up to 62% reduced rate of adequate antibody response, while those on non-MTX medications demonstrated levels of immunogenicity comparable to HCs [28]. Notably, this study identified decreased induction in the frequencies of activated and granzyme B-producing CD8+ T cells in participants with IMID on MTX. Robust expansion of functional CD8+ T cells early after COVID-19 vaccination has been suggested to play important roles in protection against SARS-CoV-2 infection [29]. Studies in non-human primates suggest that CD8+ T cells contribute to protection against rechallenge with SARS-CoV-2, especially in the setting of waning antibody titers [30]. Taken together, these suggest that MTX may impair the immunogenicity of COVID-19 vaccine by blunting both cellular and humoral responses.
Four other cohort studies on immune responses against SARS-CoV-2 vaccination among patients with AIIRD further confirmed a lower seroconversion rate in those being treated with MTX [14, 19, 21, 31]. However, a larger cohort study by Boekel et al. demonstrated a significantly lower rate of seroconversion in AIIRD patients without previous SARS-CoV-2 infection as compared to healthy controls following the first dose of SARS-CoV-2 vaccination, while the seroconversion rate reached similar after the second dose. This discrepancy may be explained the relatively long median time between vaccination and sampling in the patient cohort (38 days), which suggests that MTX might delay rather than impair the development of humoral immunity [15].
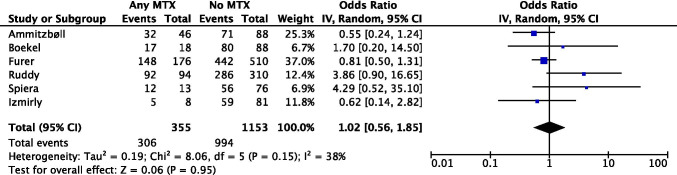
Our meta-analysis (Fig 3) including 6 observational studies (pooled n=1508) did not show an association between MTX use and a lower seroconversion rate following SARS-CoV-2 vaccination in patients with AIIRDs (pooled OR=1.02 [95% CI 0.56, 1.85]). It is not unexpected as the patients were highly heterogeneous with different autoimmune disorders and on various immunosuppressive regimens other than MTX monotherapy. For example, patients identified as “No MTX” might be on RTX or MMF, which would have significantly reduced their immune responses against vaccination, thus confounding the result. Additionally, it is possible that some patients held their MTX around vaccination based on the American College of Rheumatology (ACR) COVID-19 vaccine clinical guidance.
Fig 3.
Meta-analysis of 6 observational studies investigating methotrexate use and seroconversion following SARS-CoV-2 vaccination
Mycophenolate or Mycophenolic Acid
A 2013 case-control study that investigated the immunogenicity of quadrivalent HPV vaccine in SLE patients reported that patients on MMF had lower seroconversion rates for two genotypes than those on other immunomodulators and anti-HPV titers were inversely correlated with MMF dose [32]. Solid organ transplant recipients treated with MMF-based regimens were reported to have higher incidence of impaired humoral responses to SARS-CoV-2 BNT162b2 mRNA vaccine [33, 34]. In line with these, five cohort studies showed reductions in the seroconversion rate following SARS-CoV-2 vaccination in AIIRD patients treated with MMF-based regimens [14, 19, 21, 24, 35].
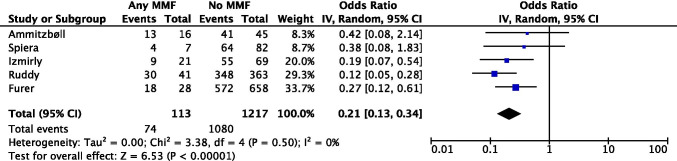
Our meta-analysis (Fig 4) including 5 observational studies (pooled n=1330) showed an association between MMF use and a lower seroconversion rate following SARS-CoV-2 vaccination among patients with AIIRDs (pooled OR=0.21 [95% CI 0.13, 0.34]).
Fig 4.
Meta-analysis of 5 observational studies investigating mycophenolate/mycophenolic acid use and seroconversion following SARS-CoV-2 vaccination in AIIRD patients
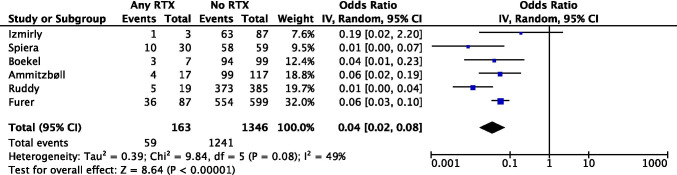
Rituximab
RTX depletes CD20+ B cells and diminishes humoral immune responses to vaccination. Substantial reductions in immune responses to SARS-CoV-2 vaccinations have been consistently observed in AIIRD patients treated with RTX across all studies [14, 16, 19, 21, 24, 35–37].
Spiera et al. reported that longer duration from last RTX exposure was associated with higher rates of seroconversion and that even low levels of B-cell reconstitution was sufficient to render more robust immune responses, illustrating the importance of appropriate timing when administering SARS-CoV-2 vaccination in RTX-exposed AIIRD patients [37]. Furthermore, Deepak et al. found that the reduction in antibody titers and neutralization activities were most significant when SARS-CoV-2 vaccination occurred within 6 months of BCDT, with gradual recovery after 9-month post-treatment with RTX [21]. However, Salviani et al. described 2 patients with AAV who failed to develop neutralizing antibodies after SARS-CoV-2 mRNA vaccines even though the most recent RTX infusion was more than 9 months earlier [38]. Furthermore, Ammitzbøll et al. found a strikingly low seroconversion rate in RA and SLE patients (n=17) treated with RTX (4/17, 23%) after two doses of BNTb262 mRNA vaccine. The median time interval between last RTX infusion and vaccination was 187 (IQR 150–245) days, and this time interval did not influence levels of total antibody against SARS-CoV-2 [16].
Our meta-analysis (Fig 5) including 6 observational studies (pooled n=1509) revealed a significant association between RTX use and a lower seroconversion rate following SARS-CoV-2 vaccination among patients with AIIRDs (pooled OR=0.04 [95% CI 0.02, 0.08]).
Fig 5.
Meta-analysis of 6 observational studies investigating rituximab use and seroconversion following SARS-CoV-2 vaccination in AIIRD patients
Safety of COVID-19 Vaccine in Patients with Systemic Lupus Erythematosus
The pathogenesis of SLE and COVID-19 rely on the activation of similar molecular networks, including type I interferon and proinflammatory cytokine pathways [39, 40]. The nucleic acid-based vaccines used for COVID-19 can activate these pathways by signaling through endosomal toll-like receptors (TLRs) and cytosolic RNA and DNA sensors (such as MDA5, RIG-I, and cGAS) that trigger inflammasome activation and type I interferon production, raising initial concern that such approaches could induce disease flares [41]. In addition, we previously speculated that the mRNA vaccines may interact with cytoplasmic RNA-binding proteins involved in the post-translational regulation of inflammatory mediators, thus triggering autoimmune disease activity [9]. Transient elevations of various autoantibodies were observed in patients with SLE following influenza vaccinations though these elevations were not consistently associated with increase in disease activity [42, 43]. Felten et al. conducted a web-based survey in December 2020 on the expectations and concerns regarding SARS-CoV-2 mRNA vaccines in patients with AIIRD (n=1266). Roughly half of the participants expressed unwillingness (13.2%) and uncertainty (32.2%) to get vaccinated; scarcity of vaccine safety profiles and possible induction of a disease flare were identified among the main concerns leading to vaccine hesitancy [44].
Despite all the above theoretical concerns, current data on the safety of COVID-19 vaccines in patients with AIIRDs have been reassuring albeit scarce (Table 2).
Table 2.
Summary of studies on COVID-19 vaccine safety in patients with autoimmune inflammatory rheumatic disorders
| Author | Design | Vaccine | Cohort | Diseases | Therapy | Time period | Results |
|---|---|---|---|---|---|---|---|
| Geisen et al. | Cohort | Pfizer, Moderna |
26 AIIRDs 42 HCs |
SLE 2 (7.7%), RA 8 (30.1%), PsA 6 (23.1%), SpA 3 (11.5%) | GC 7 (26.9%), TNFi 13 (50%), cDMARDs 8 (30.8%) | Pre 1st dose to 14 days post 2nd dose | Stable DAS28, PGA, PhGA pre- and post-vaccine No patient with AIIRDs needed to adjust DMARD or GC therapy in the 6 weeks of trial duration |
| Izmirly et al. | Cohort | Pfizer, Moderna, J&J |
90 SLE 20 HCs |
SLE specific | GC 26 (29%), MTX 8 (14.9%), MMF 21 (23%), RTX 3 (3%), BLyS 10 (11%) | Average 23.6 days (range 5–70) post final dose | Similar pre-/post-vaccine SLEDAI 9 (11.4%) patients had a post-vaccination flare; 8/9 mild/moderate and 1/9 severe No changes in % (+) anti-dsDNA and/or low C3/C4 |
| Furer et al. | Cohort | Pfizer | 686 AIIRD | SLE 101 (14.7%); RA 263 (38.3%), PsA 165 (24.1%) | GC 55 (21%), MTX 116 (44%), TNFi (18%), BCDT 87 (13%), MMF 38 (4%) | Within 2 weeks post 1st dose, within 2–6 weeks post 2nd dose | Stable pre- and post-vaccine disease activity indices |
| 121 HC | |||||||
| Conolly et al. | Cohort | Pfizer, Moderna | 325 AIIRD | SLE 91 (28%), IA 123 (38%), CTDs 62 (19%) | csDMARDs 63 (44%), biologics 62 (19%), combination 120 (37%) | Within 1st week post 1st dose | Peripheral neuropathy—1 case Local side effects—89%, pain most common Systemic side effects—69%, fatigue most common. Similar symptoms to those reported in general population vaccine trials |
| Boekel et al. | Cross-sectional | Pfizer, AZ, Moderna |
505 IMID 203 HC |
SLE 25 (5%), RA 204 (40%), PsA 49 (10%); AS 39 (8%), MS 81 (16%), PMR 17 (3%) | No treatment 157 (31%), MTX 169 (33%), other csDMARDs 67 (13%), GC 77 (15%), TNFi 93 (18%), BCDT 35 (7%) | Median of 15 days (IQR 10–25) for AIIRD and 15 days (10–34) for HC post 1st dose | Adverse events of COVID-19 vaccines in patients with IMID are comparable with controls Patients with AIIRD more frequently reported joint pain than controls (10% vs 1%) 26 (5%) patients with AIIRDs self-reported disease worsening up to 2 months post vaccine |
| Felten et al. | Cross-sectional | Mixed types | 696 SLE | SLE specific | GC 373 (53.6%), HCQ 542 (77.9%), MTX/LEF/MMF/AZA/CTX 347 (49.9%), BLyS 76 (10.9%); RTX 22 (3.2%) | Not applicable | 21 (3%) self-reported/physician confirmed SLE flares after a median of 3 days (IQR 0–29) post vaccine; 15 treatment change, and 4 admissions for SLE flares. Flare in the year pre-vaccine was associated an increased risk of post-vaccine flare (RR =5.52, 95% CI 2.17–14.03). |
| Watad et al. | Case series | Pfizer, AZ, Moderna | 17 flares, 10 de-novo IMIDs | 4 cases in SLE | Not applicable | Not applicable | 4 cases of SLE flares |
Pfizer, Pfizer/BioNTech BNT162b2; Moderna, Moderna mRNA-1273; J&J, Janssen Ad26.COV2.S; AZ, AstraZeneca ChAdOx1 nCoV-19
IMID immune-mediated inflammatory disease, AIIRD autoimmune inflammatory rheumatic diseases, HC healthy control, SLE systemic lupus erythematosus, RA rheumatoid arthritis, SpA spondyloarthritis, PsO psoriasis, PsA psoriatic arthritis, AS ankylosing spondyloarthritis, PMR polymyalgia rheumatica, MS multiple sclerosis, IA inflammatory arthritis, CTD overlap connective tissue disorders, csDMARDs conventional synthetic disease-modifying antirheumatic drugs, HCQ hydroxychloroquine, GC glucocorticoid, MTX methotrexate, AZA azathioprine, LEF leflunomide, MMF mycophenolate mofetil, CTX cyclophosphamide, RTX rituximab, BCDT B-cell depletion therapy, BLyS belimumab, TNFi tumor necrosis factor inhibitor, % percentage of patients, (+) positive, Anti-dsDNA anti-double stranded DNA antibody, IQR interquartile range, RR relative risk, CI confidence interval, DAS28 disease activity score 28, PGA Patients Global Assessment, PhGA Physician Global Assessment, SLEDAI Systemic Lupus Erythematosus Disease Activity Index, SDAI Simple Disease Activity Index, DAPSA Disease Activity Index for Psoriatic Arthritis, PASI Psoriasis Area and Severity Index, ASDAS Ankylosing Spondylitis Disease Activity Score
Concerns for Disease Flare
Two studies focused on the adverse events following the first dose of COVID-19 vaccine in patients with AIIRD [36, 45]. Reassuringly, both reported that side effect profiles in patients with AIIRD were comparable to data generated from the general population and to large-scale vaccine trials, with high prevalence of reactogenicity, among which pain and fatigue were the most common local and systemic symptoms, respectively. Conolly et al. did note one case of peripheral neuropathy during the 1-week follow-up of 325 patients [45]. Boekel et al. did, however, report a small proportion of 26 patients (5%) with AIIRD that experienced worsening of their underlying disease up to 2 months after vaccination.
A couple of studies evaluated the disease activity of underlying autoimmune disorders in patients with AIIRD before and after two doses of SARS-CoV-2 mRNA vaccines [19, 46]. Geisen et al. monitored disease activity of patients with AIIRD (n=26) by Patient Global Assessment (PGA) plus Physician Global Assessment (PhGA) and additionally monitored patients with inflammatory arthritis (n=8) by Disease Activity Score-28 (DAS-28) [46]. No disease flares were observed following either vaccination time point, and no changes in use of GC or DMARDs were needed during the 6-week trial duration. Similarly, the above cohort study by Furer et al. reported stable post-vaccination indices of disease activity across different disease types, including SLE (measured by SLE Disease Activity Index [SLEDAI]) [19].
Another two studies investigated the safety profiles of COVID-19 vaccine specifically in patients with SLE [14, 47]. Felten et al. surveyed a group of patients with self-reported medically confirmed SLE (n=696) for the occurrence of side effects. Twenty-one (3%) patients reported a medically confirmed SLE flare after a median IQR of 3 days (0–29) following vaccination, which led to treatment change in 15 (71%) and hospital admission in 4 (19%) patients; notably, the presence of a SLE flare in the year preceding vaccination was identified as a risk factor for post-vaccination disease flare [47]. In the study by Izmirly et al., SLE patients (n=90) were also assessed for pre- and post-vaccination disease activities by clinical and serological parameters. SLEDAI scores, percentages of patients with abnormal anti-double-stranded DNA (anti-dsDNA) antibodies and/or abnormal complement levels, and levels of C3 and C4 remained similar pre- and post-vaccination. Nine (11.4%) patients had post-vaccination flares, of which only one was severe and characterized by arthritis requiring MTX treatment [14].
Notably, Watad et al. presented a series of 27 cases including 17 disease flares and 10 de novo onsets of AIIRDs following administration of SARS-CoV-2 vaccines. Among them were two SLE flares and one case of discoid SLE that converted to pleuropericarditis [48].
Thrombosis and Thrombocytopenia
Rare development of unusual thrombotic events and thrombocytopenia have been observed within 1–2 weeks of vaccination with adenoviral vector SARS-CoV-2 vaccines, ChAdOx1 nCov-19 (AstraZeneca) and Ad.26.COV2.S (Janssen). The majority of cases occurred in women, predominantly involved cerebral venous sinuses, and presented clinical pictures as well as serological reactivity strongly resembling those of autoimmune heparin-induced thrombocytopenia [49].
These cases led to a temporary pause on the administration of Ad.26.COV2.S (Janssen) in the USA, which was later lifted due to the known benefits of protection against SARS-CoV-2 infection outweighing the risks, as determined by FDA and CDC advisory committees. However, no reports of thrombosis and thrombocytopenia were noted in SLE patients.
Discussion
As of September 1, 2021, more than 686 million doses of COVID-19 vaccine have been delivered across the USA, and 61.9% of the total population has received at least one dose of vaccine [50]. Despite limited data on the efficacy and safety profiles in patients with AIIRD, the majority of this population has been fully vaccinated in accordance with ACR COVID-19 vaccine clinical guidance [11]. Our review and analyses show that patients with SLE are less likely to generate adequate immune responses, to COVID-19 vaccines, an effect likely driven by immunosuppressants commonly used to treat SLE, including GC, MTX, MMF, and RTX can further delay or impair vaccine responses. Reassuringly, there is no major safety concern regarding the use of COVID-19 vaccine in patients with AIIRD.
Several critical questions remain unanswered. First of all, previous studies on the efficacy of COVID-19 vaccination in SLE patients invariably focused on the immediate immune response as measured by antibody titers, neutralization activities, and, in some studies, T cell responses. However, no studies have assessed the durability of vaccine responses in SLE patients. Second, emerging SARS-CoV-2 variants, with high transmissibility, extreme virulence, and ability to evade vaccine-induced immunity, have up-trended the COVID-19 case number in recent months with a considerable proportion of fully vaccinated people being re-infected [51]. As a result, over 1 million people chose to get a booster vaccine dose during the period of August 13 to September 1, according to official COVID data tracker [50]. Unfortunately, no evidence exists as to whether fully vaccinated SLE patients are at an increased risk of becoming infected with emerging variants, whether they would benefit from a booster dose, and if so, when is the optimal time to administer the booster and how to adjust the immunosuppressive regimen to optimize booster effects. We are looking forward to the results from the ACV01 study, a randomized clinical trial investigating booster effects with different immunosuppressive regimens in AIIRD patients, to further clarify these critical questions [12].
In sum, as of the date of this review, the benefits of COVID-19 vaccine against SARS-CoV-2 infection far outweighs the risks of side effects or disease flares. Therefore, we stand firmly in line with ACR guidance that all AIIRD patients should receive COVID-19 vaccine and certain immunosuppressive therapies should be modified around the time of vaccination [11].
Author Contribution
All authors contributed to the study conception and design. Anca Askanase formed the idea for the article; Wei Tang, Leila Khalili, and Edd Ricker performed the literature search; Wei Tang performed the data analysis; Leila Khalili, Yevgeniya Gartshteyn, Shane Murray, and Sean Inzerillo critically revised the work. The first draft of the manuscript was written by Wei Tang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability
Not applicable.
Code Availability
Not applicable
Declarations
Ethics Approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Systemic Lupus Erythematosus
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heaton PM. The Covid-19 Vaccine-Development Multiverse. N Engl J Med. 2020;383(20):1986–1988. doi: 10.1056/NEJMe2025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadoff J, Gray G, Vandebosch A, Cardenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FDA Approves First COVID-19 Vaccine. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine; 08/23/2021.
- 6.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Ruiz R, Paredes JL, Niewold TB. COVID-19 in patients with systemic lupus erythematosus: lessons learned from the inflammatory disease. Transl Res. 2021;232:13–36. doi: 10.1016/j.trsl.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strangfeld A, Schafer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80(7):930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang W, Askanase AD, Khalili L, Merrill JT. SARS-CoV-2 vaccines in patients with SLE. Lupus Sci Med. 2021;8(1). doi: 10.1136/lupus-2021-000479. [DOI] [PMC free article] [PubMed]
- 10.Boekel L, Hooijberg F, van Kempen ZLE, Vogelzang EH, Tas SW, Killestein J, et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. 2021;3(4):e241–e243. doi: 10.1016/S2665-9913(21)00037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of Rheumatology Guidance for COVID-19 Vaccination in Patients With Rheumatic and Musculoskeletal Diseases: Version 3. Arthritis Rheumatol. 2021 doi: 10.1002/art.41928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.COVID-19 Booster Vaccine in Autoimmune Disease Non-Responders. https://ClinicalTrials.gov/show/NCT05000216.
- 13.Puges M, Biscay P, Barnetche T, Truchetet ME, Richez C, Seneschal J, et al. Immunogenicity and impact on disease activity of influenza and pneumococcal vaccines in systemic lupus erythematosus: a systematic literature review and meta-analysis. Rheumatology (Oxford) 2016;55(9):1664–1672. doi: 10.1093/rheumatology/kew211. [DOI] [PubMed] [Google Scholar]
- 14.Izmirly PM, Kim MY, Samanovic M, Fernandez-Ruiz R, Ohana S, Deonaraine KK, et al. Evaluation of immune response and disease status in SLE patients following SARS-CoV-2 Vaccination. Arthritis Rheumatol. 2021. doi: 10.1002/art.41937. [DOI] [PMC free article] [PubMed]
- 15.Boekel L, Steenhuis M, Hooijberg F, Besten YR, van Kempen ZLE, Kummer LY, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed]
- 16.Ammitzboll C, Bartels LE, Bogh Andersen J, Risbol Vils S, Elbaek Mistegard C, Dahl Johannsen A, et al. Impaired antibody response to the BNT162b2 messenger RNA coronavirus disease 2019 vaccine in patients with systemic lupus erythematosus and rheumatoid arthritis. ACR Open Rheumatol. 2021. doi: 10.1002/acr2.11299 [DOI] [PMC free article] [PubMed]
- 17.Ghione P, Gu JJ, Attwood K, Torka P, Goel S, Sundaram S, et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell directed therapies. Blood. 2021. doi: 10.1182/blood.2021012443 [DOI] [PMC free article] [PubMed]
- 18.Simon D, Tascilar K, Fagni F, Kronke G, Kleyer A, Meder C, et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis. 2021. doi: 10.1136/annrheumdis-2021-220461 [DOI] [PMC free article] [PubMed]
- 19.Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021. doi: 10.1136/annrheumdis-2021-220647 [DOI] [PubMed]
- 20.Rondaan C, Furer V, Heijstek MW, Agmon-Levin N, Bijl M, Breedveld FC, et al. Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: a systematic literature review for the 2019 update of EULAR recommendations. RMD Open. 2019;5(2):e001035. doi: 10.1136/rmdopen-2019-001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, Demissie EG, et al. Effect of Immunosuppression on the immunogenicity of mRNA Vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med. 2021 doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer L, Gerstel PF, Poncet A, Siegrist CA, Laffitte E, Gabay C, et al. Pneumococcal polysaccharide vaccination in adults undergoing immunosuppressive treatment for inflammatory diseases–a longitudinal study. Arthritis Res Ther. 2015;17:151. doi: 10.1186/s13075-015-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borba EF, Saad CG, Pasoto SG, Calich AL, Aikawa NE, Ribeiro AC, et al. Influenza A/H1N1 vaccination of patients with SLE: can antimalarial drugs restore diminished response under immunosuppressive therapy? Rheumatology (Oxford) 2012;51(6):1061–1069. doi: 10.1093/rheumatology/ker427. [DOI] [PubMed] [Google Scholar]
- 24.Ruddy JA, Connolly CM, Boyarsky BJ, Werbel WA, Christopher-Stine L, Garonzik-Wang J, et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021. doi: 10.1136/annrheumdis-2021-220656 [DOI] [PMC free article] [PubMed]
- 25.Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti-tumor necrosis factor alpha, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2014;66(7):1016–1026. doi: 10.1002/acr.22246. [DOI] [PubMed] [Google Scholar]
- 26.Park JK, Lee MA, Lee EY, Song YW, Choi Y, Winthrop KL, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2017;76(9):1559–1565. doi: 10.1136/annrheumdis-2017-211128. [DOI] [PubMed] [Google Scholar]
- 27.Park JK, Lee YJ, Shin K, Ha YJ, Lee EY, Song YW, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77(6):898–904. doi: 10.1136/annrheumdis-2018-213222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haberman RH, Herati R, Simon D, Samanovic M, Blank RB, Tuen M, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021. doi: 10.1136/annrheumdis-2021-220597 [DOI] [PMC free article] [PubMed]
- 29.Oberhardt V, Luxenburger H, Kemming J, Schulien I, Ciminski K, Giese S, et al. Rapid and stable mobilization of CD8(+) T cells by SARS-CoV-2 mRNA vaccine. Nature. 2021;597(7875):268–273. doi: 10.1038/s41586-021-03841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630–634. doi: 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Janabi A, Littlewood Z, Griffiths CEM, Hunter HJA, Chinoy H, Moriarty C, et al. Antibody responses to single-dose SARS-CoV-2 vaccination in patients receiving immunomodulators for immune-mediated inflammatory disease. Br J Dermatol. 2021 doi: 10.1111/bjd.20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mok CC, Ho LY, Fong LS, To CH. Immunogenicity and safety of a quadrivalent human papillomavirus vaccine in patients with systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 2013;72(5):659–664. doi: 10.1136/annrheumdis-2012-201393. [DOI] [PubMed] [Google Scholar]
- 33.Peled Y, Ram E, Lavee J, Sternik L, Segev A, Wieder-Finesod A, et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transplant. 2021;40(8):759–762. doi: 10.1016/j.healun.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben-Yehoyada M, Shashar M, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyarsky BJ, Ruddy JA, Connolly CM, Ou MT, Werbel WA, Garonzik-Wang JM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80(8):1098–1099. doi: 10.1136/annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boekel L, Kummer LY, van Dam KPJ, Hooijberg F, van Kempen Z, Vogelzang EH, et al. Adverse events after first COVID-19 vaccination in patients with autoimmune diseases. Lancet Rheumatol. 2021;3(8):e542–e545. doi: 10.1016/S2665-9913(21)00181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiera R, Jinich S, Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis. 2021. doi: 10.1136/annrheumdis-2021-220604 [DOI] [PubMed]
- 38.Spiera R, Jinich S, Jannat-Khah D. Rituximab, but not other antirheumatic therapies, is associated with impaired serological response to SARS- CoV-2 vaccination in patients with rheumatic diseases. Ann Rheum Dis. 2021. doi: 10.1136/annrheumdis-2021-220604 [DOI] [PubMed]
- 39.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Postal M, Vivaldo JF, Fernandez-Ruiz R, Paredes JL, Appenzeller S, Niewold TB. Type I interferon in the pathogenesis of systemic lupus erythematosus. Curr Opin Immunol. 2020;67:87–94. doi: 10.1016/j.coi.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiesik-Szewczyk E, Romanowska M, Mielnik P, Chwalinska-Sadowska H, Brydak LB, Olesinska M, et al. Anti-influenza vaccination in systemic lupus erythematosus patients: an analysis of specific humoral response and vaccination safety. Clin Rheumatol. 2010;29(6):605–613. doi: 10.1007/s10067-010-1373-y. [DOI] [PubMed] [Google Scholar]
- 43.Abu-Shakra M, Press J, Sukenik S, Buskila D. Influenza virus vaccination of patients with SLE: effects on generation of autoantibodies. Clin Rheumatol. 2002;21(5):369–372. doi: 10.1007/s100670200099. [DOI] [PubMed] [Google Scholar]
- 44.Felten R, Dubois M, Ugarte-Gil MF, Chaudier A, Kawka L, Bergier H, et al. Vaccination against COVID-19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021;3(4):e243–e245. doi: 10.1016/S2665-9913(21)00039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL, et al. Safety of the first dose of mRNA SARS-CoV-2vaccines inpatients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geisen UM, Berner DK, Tran F, Sumbul M, Vullriede L, Ciripoi M, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021. doi: 10.1136/annrheumdis-2021-220272 [DOI] [PMC free article] [PubMed]
- 47.Felten R, Kawka L, Dubois M, Ugarte-Gil MF, Fuentes-Silva Y, Piga M, et al. Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study. Lancet Rheumatol. 2021 doi: 10.1016/S2665-9913(21)00221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines (Basel). 2021;9(5). doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed]
- 49.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.CDC: COVID Data Tracker https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total Accessed 09/01/2021
- 51.CDC: SARS-CoV-2 Variant Classifications and Definitions. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html Accessed 09/01/2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable