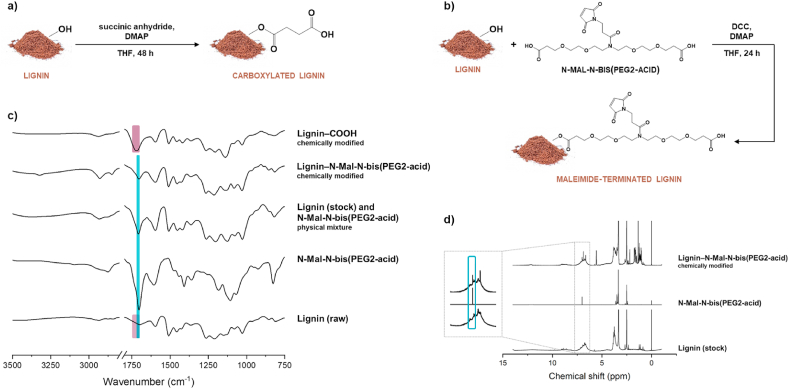
Fig. 1.
Lignin chemical modification. Schematic representation of a) lignin carboxylation reaction and b) lignin chemical modification with a maleimide-terminated heterofunctional PEG linker (N-Mal-N-bis [PEG2-acid]); c) ATR−FTIR spectra, from bottom to top, of BioPiva™ softwood kraft lignin (raw), N-Mal-N-bis(PEG2-acid), their physical mixture, lignin that was chemically modified with the maleimide-terminated N-Mal-N-bis(PEG2-acid), and carboxylated lignin; d)1H NMR spectra of lignin (stock), N-Mal-N-bis(PEG2-acid), and lignin after chemical modification with N-Mal-N-bis(PEG2-acid).