Summary
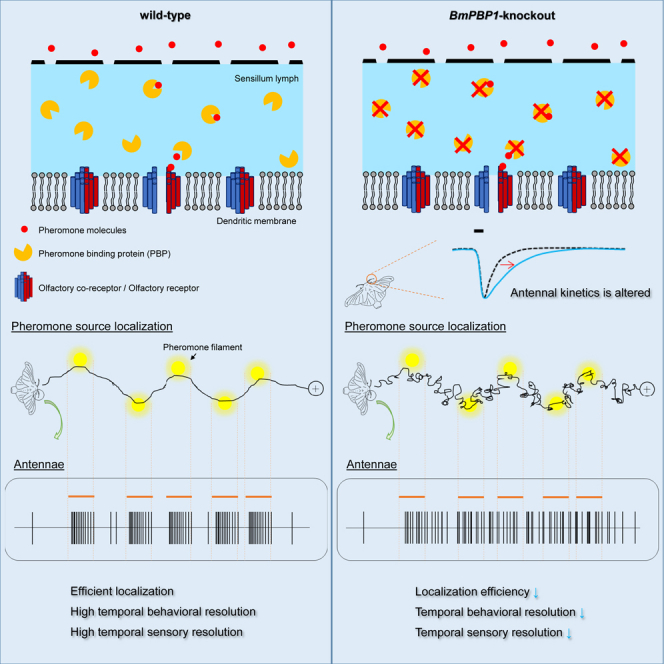
Male moths utilize spatio-temporal female sex pheromone information to orient toward conspecific females. Pheromones are distributed as discontinuous plumes owing to air turbulence; thus, efficient tracking of intermittent stimuli is expected to require a high temporal resolution. Here, using pheromone binding protein (BmPBP1)-knockout silkmoths, we showed that a loss of functional PBP lowered the temporal sensory resolution of male antennae. This altered temporal resolution resulted in significantly reduced straight walking and longer turning behavior, which respectively occurred when males detected and lost contact with pheromones, indicating that temporal resolution was also lowered at the behavioral level. BmPBP1-knockout males required significantly longer time than wild-type males in locating pheromone sources and female moths. Our results suggest that BmPBP1 plays a critical role in determining olfactory response kinetics. Accordingly, high temporal olfactory and behavioral resolutions, as shaped by PBP, are essential for tracking pheromone plumes and locating females efficiently.
Subject areas: Zoology, Entomology, Molecular mechanism of behavior
Graphical abstract
Highlights
-
•
B. mori pheromone binding protein 1 is involved in olfactory response kinetics
-
•
BmPBP1 contribute to the temporal sensory resolution
-
•
Altered sensory responses cause lower temporal behavioral resolution
-
•
Efficient localization requires high temporal olfactory and behavioral resolutions
Zoology; Entomology; Molecular mechanism of behavior
Introduction
Many animals rely on olfaction to locate potential mating partners or food sources for survival in a natural environment. However, odor source localization is a difficult task for animals as odors are discontinuously distributed owing to turbulent air flow in the natural environment, while the stochastic nature of odor distribution further increases the unpredictability of odor arrival (Murlis and Jones, 1981; Murlis et al., 1992, Murlis et al., 2000). Mechanisms underlying how animals accurately locate odor sources in such a complex environment have long represented a central topic in the field of olfactory research.
The sex pheromone communication system in moths is one of the best examples of sophisticated odor source localization in animals, whereby male moths detect and orient toward conspecific females using intermittent sex pheromone information emitted by the females. Owing to the simple relationship between sex pheromone chemicals and the searching behavior, pheromone source searching behaviors can be relatively easily induced in the laboratory. As such, this behavior has been studied as an essential model system to better understand the general mechanisms underlying odor source localization in animals.
Odor source localization in male moths consists of two basic behavioral components: upwind surge and crosswind casting (Baker, 1990). Surge refers to a straight upwind flight triggered shortly after exposure to a pheromone filament, whereas crosswind casting is a self-steering counterturning that occurs when a male moth loses contact with the pheromone filaments in a plume. In the silkmoth Bombyx mori, a fundamental strategy of flying moths is observed when the male orients itself toward a pheromone source: when briefly exposed to the pheromone, male moths exhibit a reflexive straight-line walking (surge), followed by self-steering left and right zigzag turns with a gradually increasing turn angle, and then, they perform looping (turns of more than 360° or greater) behavior. Because both zigzagging and looping are pre-programmed behaviors, these two behaviors are regarded as the same sequential behavior (we refer to the pre-programmed behavior as “zigzag turns”) (Kanzaki et al., 1992; Haupt et al., 2010; Sakurai et al., 2014; Ando and Kanzaki, 2015). This behavioral sequence is reset in response to each new pheromone stimulus. Thus, search-related walking paths are highly dependent on the temporal structure of the pheromone stimuli (Kramer, 1986, 1992; Kanzaki et al., 1992). When the stimulation frequency is low, males walk in complex zigzag paths, whereas a higher frequency results in successive surge behaviors and straighter paths. Therefore, male antennae likely possess sufficient temporal resolution to track intermittent pheromone stimulation. Indeed, this behavioral model for odor source localization is thought to be a common strategy among moth species underlying their searching behavior (Baker, 1990; Vickers and Baker, 1994).
The importance of intermittent odor information has also been highlighted in experiments using homogeneous pheromone stimulation that rarely contains fluctuating pheromone filaments in the plume (referred to as a homogeneous pheromone cloud). In homogeneous pheromone clouds, moths cannot sustain an upwind surge and exhibit a casting flight, leading to the inability to localize the pheromone source (Kennedy et al., 1980, 1981). Later, to investigate the physiological responses to intermittent stimuli, sensory response characteristics at various odor frequencies, including a homogeneous pheromone cloud, were examined in the moths Grapholita molesta and Heliothis virescens (Baker et al., 1985; Vickers and Baker, 1994). In response to intermittent stimuli, the electroantennographic (EAG) responses, which monitor the total activity of all olfactory receptor neurons (ORNs) in the antenna, of moths reflected intermittent exposure to pheromone filaments in the plume. However, in a homogeneous pheromone cloud, the responses did not show dynamic changes. These reports conclude that moths naturally receive intermittent pheromone information on their antennae, and this modulates surge-casting searching behavior. Therefore, resolving the temporal properties of odor information continuously is thought to be a very important aspect of odor source localization (Rumbo and Kaissling, 1989; Marion-Poll and Tobin, 1992).
Odorant binding proteins (OBPs) are small soluble proteins highly enriched in the sensillum lymph space (Vogt and Riddiford, 1981). These proteins contribute to the sensitivity and/or selectivity of antennal responses (Gräter et al., 2006; Große-Wilde et al., 2006; Hooper et al., 2009; Ye et al., 2017; Shiota et al., 2018). Furthermore, OBPs are considered to be involved in the rapid inactivation of pheromones, following pheromone detection. In moths, pheromone binding proteins (PBPs), a moth-specific subfamily of OBPs, participate in pheromone reception (Vogt et al., 2015). Thus, PBPs are a candidate molecular factor for controlling temporal sensory responses (Ziegelberger, 1995; Kaissling, 2001, 2009, 2013). However, this hypothesis has not been experimentally tested in vivo. In the silkmoth B. mori, one of three PBP genes named BmPBP1, which is expressed in accessory cells surrounding ORNs and secreted into the sensillum lymph of pheromone-sensitive sensilla, is reported to play an important role in pheromone detection (Sakurai et al., 2004; Maida et al., 2005; Forstner et al., 2006). In a previous study, we established a BmPBP1-knockout silkmoth line and showed that the loss of BmPBP1 affected the sensitivity of EAG responses to pheromone components (Shiota et al., 2018). In this study, using the BmPBP1-knockout silkmoth, we revealed that the temporal sensory resolution of BmPBP1-knockout male antennae to intermittent pulse trains was significantly reduced. In addition, BmPBP1-knockout male moths took significantly longer to locate pheromone sources owing to lowered temporal behavioral resolution, resulting in inefficient source localization. Based on our results, we will discuss the effects of BmPBP1 loss and the sensory characteristics that govern the efficiency of odor source searching behavior.
Results
Time constant of antennal responses was altered in BmPBP1-knockout male moths
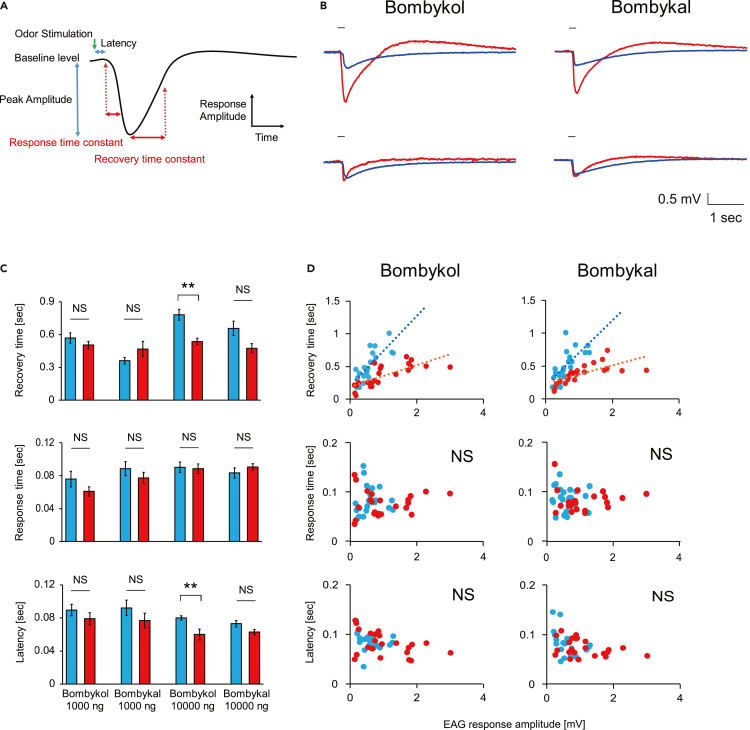
In a previous study, we reported that antennal responses of BmPBP1-knockout silkmoths to pheromone components were significantly lower than those of wild-type moths, however, the antennae retained a clear responsiveness to pheromone components (Shiota et al., 2018), (E,Z)-10,12-hexadecadien-1-ol (bombykol) (Butenandt et al., 1959), and (E,Z)-10,12-hexadecadienal (bombykal) (Kaissling et al., 1978). Using BmPBP1-knockout males, we first tested whether PBPs were also involved in the temporal properties of antennal responses by investigating the response and recovery time constants of EAG responses of BmPBP1-knockout male moth antennae to single pulse stimulation with sex pheromone components (Figure 1A). Comparison of these parameters with the same bombykol dosage (1,000 and 10,000 ng) revealed that there were significant differences in the kinetic parameters consisting of latency and recovery time constant at 10,000 ng between BmPBP1 knockouts and wild-types (Figures 1B top and 1C). Because the peak EAG amplitudes of BmPBP1-knockout male antennae were significantly lower than those of wild-type antennae (Figure 1B top) (Shiota et al., 2018), we hypothesized that differences in peak amplitudes could affect the temporal parameters of EAG responses. When we corrected the temporal parameters as a function of the peak amplitudes (see also the STAR Methods section), we found that recovery time constants were positively correlated with peak amplitudes, and, as a result, recovery time constants of BmPBP1-knockout male antennae were significantly longer than those of wild-type male antennae (p = 0.0014 for bombykal; p = 0.0017 for bombykol; Figures 1B bottom and 1D). However, response time constant and latency did not correlate with peak amplitude, and no significant differences were found in the response time constant or latency of antennal responses of BmPBP1-knockout and wild-type males (Figures 1B bottom and 1D). These differences were observed with both bombykol and bombykal (Figure 1D), suggesting that the loss of BmPBP1 delayed the recovery time constant of EAG responses with both bombykol and bombykal.
Figure 1.
Antennal response kinetics in BmPBP1-knockout male moths
(A) Parameters considered in the antennal response kinetics analysis of EAG recordings.
(B) Representative EAG responses of BmPBP1-knockout (blue) and wild-type (red) male antennae to 10,000 ng bombykol stimulation (top), and responses to 10,000 ng bombykol stimulation in BmPBP1-knockout (blue) and 100 ng bombykol stimulation in wild-type (red) male antennae (bottom). The stimulus was applied for 200 ms, as indicated by the solid line on the trace.
(C) Kinetic analysis of EAG responses in BmPBP1-knockout (blue; n = 11) and wild-type (red; n = 5) male antennae. Error bars represent ±SEM. The asterisks indicate significant differences between the groups (∗∗p < 0.01, Student’s t test for paired samples). NS indicates no significant difference.
(D) Kinetic analysis of EAG responses of BmPBP1-knockout (blue; n = 11) and wild-type (red; n = 5) antennae corrected by peak EAG amplitude. Broken lines indicate linear regression curves. An ANCOVA test was used to detect significant differences between wild-type and BmPBP1-knockout datasets for recovery time (p < 0.01), whereas significant differences between the groups were not detected in the other two parameters. NS indicates no significant difference.
Kinetics of temporal sensory responses to pheromone pulse trains in BmPBP1-knockout moths
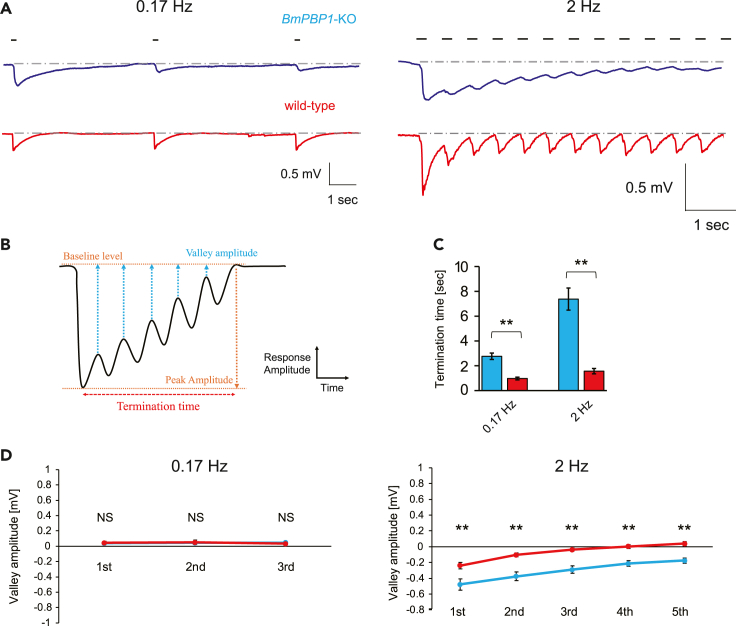
Because tracking intermittent pheromone pulse trains is important to locate pheromone sources in male moths, we next assessed the effects of loss of BmPBP1 on temporal sensory responses to pheromone pulse trains. Particularly, we focused on responses to bombykol because only bombykol triggers the pheromone source searching behavior of male silkmoths (Kaissling et al., 1978). We tested two different frequencies of pulse trains, consisting of 0.17 Hz as the low stimulus frequency and 2 Hz as the frequency that can induce a consecutive surge in male silkmoths (Figure 2A) (Kanzaki et al., 1992). Consistent with single pulse experiments, the recovery time constant of EAG responses became longer in BmPBP1-knockout than in wild-type male antennae (Figure 2A). The time required to return to baseline levels after the first bombykol stimulation (termination time) was significantly longer in BmPBP1-knockout than in wild-type male moths at both 0.17 and 2 Hz frequencies (Figures 2B and 2C). More importantly, the termination times got longer as the frequency increased and EAG responses of BmPBP1-knockout never returned to baseline levels during high-frequency stimuli in several tested antennae (4/12 tested antennae at 2 Hz). However, EAG responses of wild-type male antennae quickly recovered to baseline levels (n = 6 at 2 Hz; Figure 2C).
Figure 2.
Analysis of EAG response kinetics to pulsed bombykol stimuli in BmPBP1-knockout male moths
(A) Representative EAG responses to pulsed bombykol trains in BmPBP1-knockout (blue) and wild-type (red) male antennae at 0.17 Hz and 2 Hz. The stimulus was applied for 200 ms, as indicated by the solid line on the trace. The broken line indicates baseline level of each EAG trace.
(B) Parameters considered in the antennal response kinetics analysis of EAG recording to pulsed bombykol stimuli.
(C) Termination time of EAG responses to pulsed bombykol trains in BmPBP1-knockout (blue) and wild-type (red) antennae at 0.17 Hz (KO; n = 9, WT; n = 9) and 2 Hz (KO; n = 12, WT; n = 6). Error bars represent ±SEM. The asterisks indicate significant differences between the groups (∗∗p < 0.01, Student’s t test for paired samples).
(D) Valley amplitude of male antennae to bombykol stimuli in BmPBP1-knockout (blue) and wild type (red) at 0.17 Hz (KO; n = 9, WT; n = 9) and 2 Hz (KO; n = 16, WT; n = 7); 10,000 ng bombykol was used in BmPBP1-knockout and 100 ng of bombykol was used in wild-type males. Error bars represent ±SEM. The asterisks indicate significant differences between the groups (∗∗p < 0.01, Student’s t test for paired samples). NS indicates no significant difference.
See also Figure S1.
To evaluate response recovery after intermittent stimuli, we calculated the valley amplitudes, which were defined as the EAG amplitude where each local minimum amplitude was subtracted from the baseline level amplitude (Figures 2B and 2D, see also STAR Methods section). The valley amplitudes of BmPBP1-knockout males were significantly larger than those of wild-type males when stimuli were delivered at 2 Hz. In comparison, the valley amplitudes of both BmPBP1-knockout and wild-type males returned to baseline levels after each stimulus was delivered at 0.17 Hz, and there was no significant difference between the groups (Figure 2D). We also tested 0.83 Hz stimuli as the estimated emission rate of pheromone from female moths (Fujiwara et al., 2014). Similar to 2-Hz stimuli, two response characteristics, longer termination time and larger valley amplitudes, were observed in BmPBP1-knockout males in response to 0.83 Hz stimuli (Figure S1). These results indicate that delayed recovery time constant in BmPBP1-knockout male antennae affects the overall temporal response kinetics of EAG responses to intermittent pheromone stimuli.
Temporal resolution of a single ORN in BmPBP1-knockout male moths was lower compared with that in wild-type male moths
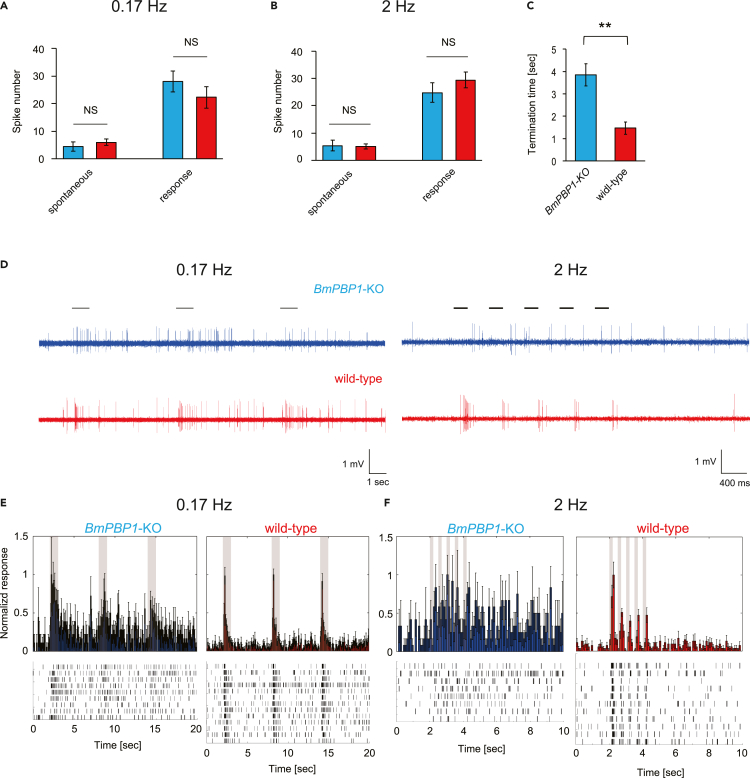
To understand the physiological basis of the observed temporal characteristics in EAG experiments, we next performed single sensillum recordings (SSRs) of pheromone-sensitive sensilla trichodea (Figure 3). We revealed that spontaneous spike activity was not significantly different between BmPBP1-knockout and wild-type antennae, suggesting that the loss of BmPBP1 does not affect spontaneous spike levels (Figures 3A and 3B). To compare temporal kinetics, we used 10,000 ng bombykol as stimuli for BmPBP1-knockout male antennae and 1,000 ng bombykol as stimuli for wild-type male antennae, which evoked similar spike counts in BmPBP1-knockout male antennae and wild-type male antennae (Figures 3A and 3B). The spike responses of BmPBP1-knockout male antennae at 0.17 Hz took significantly longer to return to spontaneous spike levels, whereas those of wild-type antennae quickly terminated after the stimulus (Figure 3C). When antennae were stimulated at 2 Hz, this tendency became clearer, and the spike responses of all tested ORNs in BmPBP1-knockout antennae did not return to spontaneous spike levels between subsequent stimuli, whereas wild-type ORN spike responses quickly returned to spontaneous levels (Figures 3E and 3F). These results demonstrate that the loss of BmPBP1 delayed response termination of ORNs resulting in a lower sensory temporal resolution in BmPBP1-knockout males.
Figure 3.
Analysis of antennal temporal resolution in single-cell spike responses to pheromone pulse trains in BmPBP1-knockout male moths
(A) Comparison of spontaneous and elicited spike numbers. Spontaneous spikes were calculated with a time bin of 2 s before the first stimulus was presented to BmPBP1-knockout (blue; n = 9 at 0.17 Hz, n = 8 at 2 Hz) and wild-type (red; n = 13 at 0.17 Hz, n = 11 at 2 Hz) male antennae; spike numbers were calculated with a time bin of 5 s from the stimulus at 0.17 Hz and (B) with a time bin of 3.5 s after the stimulus at 2 Hz. Error bars represent ±SEM. NS indicates no significant difference according to Student’s t test for paired samples.
(C) Comparison of the termination time of spikes between BmPBP1-knockout and wild-type at 0.17 Hz. Error bars represent ±SEM. The asterisks indicate significant differences between the groups (∗∗p < 0.01, Student’s t test for paired samples).
(D) Representative spike responses of long sensilla trichodea in BmPBP1-knockout (blue) and wild-type (red) male antennae to pheromone pulse stimuli at 0.17 and 2 Hz. Solid lines on the trace represent the timing of intermittent bombykol stimuli.
(E and F) (E) Spike rate in time bins of 100 ms in BmPBP1-knockout (blue; n = 9) and wild-type (red; n = 13) male antennae at 0.17 Hz and (F) those of 100 ms in BmPBP1-knockout (blue; n = 8) and wild-type (red; n = 11) male antennae at 2 Hz. Error bars represent ±SEM. 10,000 ng bombykol is used as stimuli for BmPBP1-knockout male antennae and 1,000 ng bombykol is used as stimuli for wild-type male antennae.
Temporal behavioral resolution of BmPBP1-knockout male moths was lower compared with that of wild-type male moths
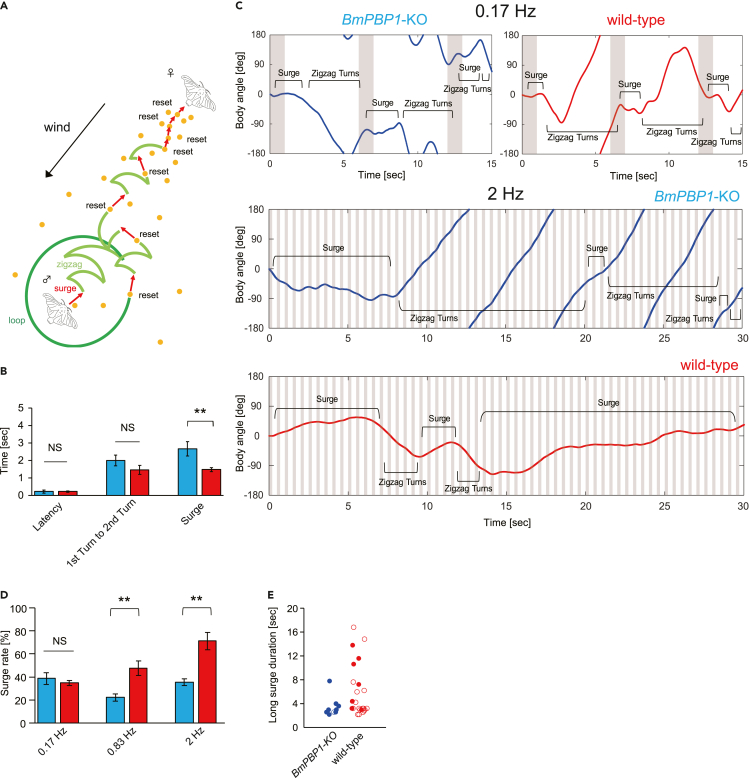
To investigate the effects of delayed termination kinetics on pheromone source searching behaviors (Figure 4A), we first analyzed pheromone source searching behaviors to bombykol using tethered male moths. Upon exposure to a single pulse of bombykol, each BmPBP1-knockout male moth exhibited full pheromone-induced searching behavior, consisting of a surge behavior followed by zigzag turns (Figures 4B and S2), indicating that the loss of BmPBP1 did not abolish innate behavioral sequences, despite the surge duration of BmPBP1-knockout male moths being significantly longer than that of wild-type male moths (Figure 4B).
Figure 4.
Behavioral responses of tethered BmPBP1-knockout moths to intermittent pheromone pulses
(A) Schematic diagram of the pheromone-triggered programmed behavior and pheromone source localization strategy of a male silkmoth, B. mori. The dots denote pheromone filaments. Note that the actual behavior during zigzagging and looping consists of point turns.
(B) Analysis of pheromone-triggered programmed behavior in BmPBP1-knockout (blue; n = 9) and wild-type (red; n = 16) male moths to single-pulse bombykol stimulation (1 s duration). Error bars represent ±SEM. The asterisks indicate significant differences between the groups (∗∗p < 0.01, Student’s t test for paired samples). NS indicates no significant difference.
(C) Representative trace of the body angle of tethered BmPBP1-knockout (blue) and wild-type male (red) moths to pulsed bombykol stimuli at 0.17 and 2 Hz.
(D) Surge rate in BmPBP1-knockout (blue) and wild-type (red) male moths to bombykol pulse trains at 0.17 Hz (KO; n = 9, WT; n = 16), 0.83 Hz (KO; n = 10, WT; n = 7), and 2 Hz (KO; n = 9, WT; n = 9). Error bars represent ±SEM. The asterisks indicate significant differences between the groups (∗∗p < 0.01, Student’s t test for paired samples). NS indicates no significant difference.
(E) Scatterplot of each surge based on surge duration in BmPBP1-knockout (blue; n = 9) and wild-type (red; n = 9) male moths. Circles indicate the distribution of individual data. Solid circles indicate the first surge that was induced by the first bombykol stimuli.
See also Figure S2.
If BmPBP1-knockout males perceived intermittent stimuli as continuous signals owing to delayed response kinetics, we hypothesized that behavioral patterns to pulse trains should be changed, especially in terms of resetting behavior sequences. In response to 0.17 Hz intermittent bombykol stimulation, to which BmPBP1-knockout antennae return to baseline levels, both BmPBP1-knockout and wild-type male moths showed a surge in exposure to each bombykol pulse (Figures 4C and 4D), indicating that each pulse induced the resetting of behavior sequences. However, following 0.83 and 2 Hz bombykol pheromone pulse trains, BmPBP1-knockout male moths displayed lower surge rates (Figures 4C and 4D)—the ratio of surge duration against total locomotion duration (see STAR Methods)—and higher zigzag turn rates than wild-type male moths (Figure 4C). Furthermore, BmPBP1-knockout male moths exhibited fewer longer surges (defined as a surge lasting more than 2 s) than wild-type male moths (Figure 4E), indicating that BmPBP1-knockout males failed to exhibit successive surge behaviors in response to intermittent pheromone stimuli at 2 Hz. Importantly, differences in surge rates were well correlated with EAG and SSR results; males restarted surge behaviors to each pheromone stimulus under stimulus conditions that allowed antennal responses to return to baseline levels, presumably owing to failure to clearly detect the onset of the subsequent stimulus. Thus, our results indicate that lower temporal sensory resolution reduces temporal behavioral resolution in BmPBP1-knockout males.
Wind tunnel experiments revealed behavioral deficits in efficient pheromone source localization in BmPBP1-knockout male moths
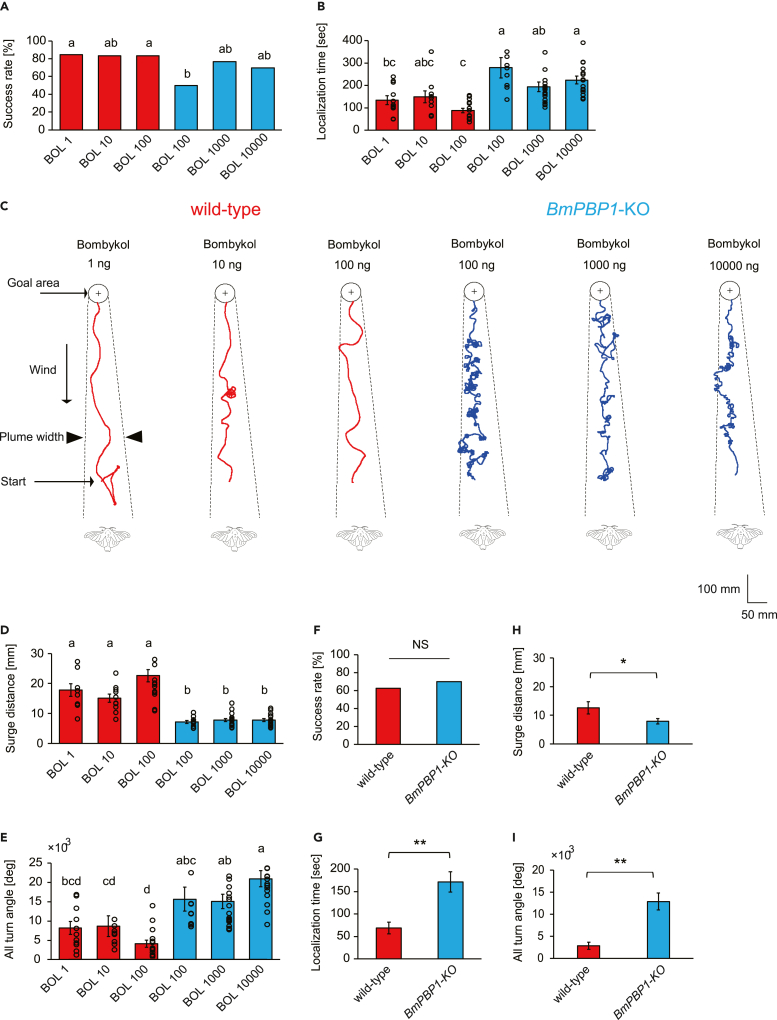
To assess whether changes in the behavioral patterns of BmPBP1-knockout males affect odor source localization, we investigated pheromone source searching behaviors to 2 Hz pheromone pulse trains in a wind tunnel under free walking conditions. We analyzed two criteria for efficient pheromone source localization: success rate and localization time. To compensate for differences in EAG sensitivity (Figure 1) and to test the effects of pheromone dose on pheromone source localization, the behaviors of BmPBP1-knockout and wild-type males were tested with 100–10,000 ng and 1–100 ng bombykol, respectively. Both BmPBP1-knockout and wild-type males could localize the pheromone source for all tested doses of bombykol with a similar success rate (Figure 5A). However, BmPBP1-knockout males took significantly longer to localize the pheromone source than wild-type males (Figure 5B). Therefore, although BmPBP1-knockout males still possess the ability to localize the pheromone sources, the efficiency of their searching behavior in terms of localization time was significantly reduced in BmPBP1-knockout males.
Figure 5.
Performance of pheromone source localization in BmPBP1-knockout male moths to bombykol
(A) Success rate of pheromone source localization of BmPBP1-knockout (blue, KO) and wild-type (red, WT) male moths at 2 Hz (WT; n = 13 at 1 ng; n = 12 at 10 ng; n = 18 at 100 ng, KO; n = 20 at 100 ng; n = 26 at 1,000 ng; n = 23 at 10,000 ng). The success rate was calculated as the number of moths that successfully localized the pheromone source divided by the total number of moths tested. The different letters indicate significant differences according to Fisher’s exact probability test.
(B) Pheromone source localization time of BmPBP1-knockout (blue) and wild-type (red) male moths at 2 Hz (KO; n = 10 at 100 ng; n = 20 at 1,000 ng; n = 17 at 10,000 ng, WT; n = 11 at 1 ng; n = 10 at 10 ng; n = 15 at 100 ng). Error bars represent ±SEM. The different letters indicate significant differences according to the Steel-Dwass test. Black open circles indicate the distribution of individual data.
(C) Representative trajectory of BmPBP1-knockout (blue) and wild-type male (red) moths to tested bombykol dosage (WT: 1, 10, 100 ng, KO: 100, 1,000, 10,000 ng) during pheromone source localization. Bombykol pulse stimuli were applied for 200 ms at 2 Hz. Solid lines indicate the trajectory of the pheromone source searching behavior. Broken lines indicate the estimated boundaries of the pheromone plume.
(D) Surge distance of BmPBP1-knockout (blue) and wild-type (red) male moth to bombykol pulse trains at 2 Hz (WT; n = 11 at 1 ng; n = 10 at 10 ng; n = 15 at 100 ng, KO; n = 10 at 100 ng; n = 20 at 1,000 ng; n = 17 at 10,000 ng). Error bars represent ±SEM. The different letters indicate significant differences according to the Steel-Dwass test. Black open circles indicate the distribution of individual data.
(E) All turn angles of BmPBP1-knockout (blue) and wild-type (red) male moths to bombykol pulse trains at 2 Hz (WT; n = 11 at 1 ng; n = 10 at 10 ng; n = 15 at 100 ng, KO; n = 10 at 100 ng; n = 20 at 1,000 ng; n = 17 at 10,000 ng). All turn angles were defined as the total cumulative turn angle during pheromone localization. Error bars represent ±SEM. The different letters indicate significant differences according to the Steel-Dwass test. Black open circles indicate the distribution of individual data.
(F–I) Performance of pheromone source localization to a female moth. Success rate (WT; n = 10, KO; n = 8), localization time (WT; n = 7, KO; n = 5), surge distance (WT; n = 7, KO; n = 5), and all turn angles (WT; n = 7, KO; n = 5) during orientation to a female moth. Error bars represent ±SEM. The asterisks indicate significant differences between the groups (∗∗p < 0.01, Student’s t test for paired samples). Significance of success rate is calculated according to Fisher’s exact probability test. NS indicates no significant difference.
The walking traces of BmPBP1-knockout males had distinct patterns with more turning and less successive surge behavior compared with wild-type males (Figure 5C; Videos S1 and S2). Indeed, a detailed analysis of the traces revealed that BmPBP1-knockout males exhibited significantly shorter surge distances and a larger summed turning angle (all turn angle) during localization than wild-type males (Figures 5D and 5E). Exposure to relatively high bombykol concentrations in BmPBP1-knockout male moths (KO: 1,000–10,000 ng) and low bombykol concentrations in wild-type male moths (WT: 1–10 ng) did not alter the characteristics of searching behaviors (Figures 5A–5E); BmPBP1-KO males showed more turning and longer localization time than wild-type males. These results indicate that the differences of the concentration of bombykol does not significantly affect the behavioral differences, thus the observed behavioral significance is not because of the differences of bombykol concentration. Lastly, we examined searching behavior to female silkmoths and found that behavioral parameters including success rate (Figure 5F), localization time (Figure 5G), surge distance (Figure 5H), and all turn angles (Figure 5I) showed the same characteristics with those observed to bombykol. Taken together, these results indicate that behavioral deficits caused by the low temporal sensory resolutions of male moths are responsible for the inefficiency of odor source localization in BmPBP1-knockout males.
Discussion
Since the discovery of PBPs in 1981 in the giant silk moth Antheraea polyphemus (Vogt and Riddiford, 1981), PBPs have been suggested to play important roles in various aspects of pheromone detection in moths. Although previous studies have provided evidence that PBPs can enhance the sensitivity of antennal response to pheromones (Ye et al., 2017; Shiota et al., 2018), no study has provided in vivo experimental evidence for the contribution of PBPs for improving temporal sensory resolution, one of the most important aspects in odor source localization under natural conditions. Using BmPBP1-knockout silkmoths, our study presents the first physiological and behavioral evidence supporting the involvement of BmPBP1 in the temporal kinetics of pheromone responses. In this study, we generated unique knockout moths with temporally altered sensory resolution but retaining programmed searching behavioral sequences comparable to wild-type moths. Our results emphasize the importance of temporal sensory response characteristics in efficient pheromone localization, which could not be tested in vivo because of the lack of such a mutant line.
Odor source localization utilizes the natural intermittency of odor stimulation to promote a surge when a stimulus pulse is encountered. This behavioral strategy for odor source localization is, at present, widely accepted in moth species, based on the correspondence between physiological antennal responses and surge behaviors during localization (Baker et al., 1985; Kramer 1986, 1992; Vickers and Baker, 1994). Therefore, moths require mechanisms that allow for the faithful representation of intermittent odor information at the level of the antennal receptors, as well as a strategy to reflect sensory information in behaviors with sufficient temporal resolution. In this study, we showed that BmPBP1-knockout males had a reduced temporal sensory resolution compared with wild-type, resulting in the inefficient localization to odor sources. Even for the localization to female silkmoths, similar behavioral deficits, such as a shorter surge distance, longer turning behavior and longer localization time, were observed in BmPBP1-knockout males. Therefore, these results indicate that moths possess an appropriate range of temporal sensory and behavioral resolutions to efficiently localize odor sources in a dynamically changing environment. Although sensitivity of BmPBP1-knockout antennae is lower than that of wild-type antennae, the behavioral deficits are thought to be caused by the alternation of temporal kinetics as the behavioral deficits were observed in response to pheromone stimuli that induced similar EAG peak amplitudes in both BmPBP1-knockout and wild-type antennae.
Our EAG results demonstrated that the recovery time of EAG responses of BmPBP1-knockout male antennae was significantly longer than those of wild-type male antennae, which resulted in the lower temporal resolution of EAG responses to pulsed pheromone stimulation. Although EAG is thought to monitor the total activity of all ORNs in the antenna, the actual relationship between EAG responses and the spike activity of individual ORNs is not clear. Thus, it is important to note that consistent temporal characteristics were also observed at the level of single ORN spike responses, showing that EAG response kinetics in BmPBP1-knockout antennae reflects changes in temporal spike characteristic of ORNs.
Similar temporal alterations in antennal and behavioral responses to odor pulse trains have been previously reported in the oriental fruit moth G. molesta (Baker et al., 1988; Linn et al., 1988). Under cool temperature conditions, antennal responses of G. molesta males to pheromones were reduced, and they were unable to keep up with high frequency pulsed pheromone stimuli due to the inability to generate well-contrasted on-off spike representations to each stimulus. This characteristic property of the spike response of ORNs is called “attenuation” (Baker et al., 1988). Under similar cooling conditions, male moths failed to initiate successive surge behaviors and could not locate an odor source (Linn et al., 1988). Although the underlying mechanism that affects the antennal response characteristics and behaviors may be different, these studies also indicate the importance of the temporal characteristics of antennal responses for efficient pheromone source localization. In our study, we revealed that BmPBP1 is one factor related to temporal sensory resolution and that temporal sensory representations corresponding to odor dynamics play a key role in the quick behavioral reset strategy for efficient localization.
In the fruit fly Drosophila melanogaster, a slower response termination kinetics, similar response characteristics observed in this study, in the antennae of Obp83a and Obp83b knockout flies has been reported (Scheuermann and Smith, 2019). However, the behavioral consequences of this altered response kinetics remained unclear. Here, using B. mori, we revealed that altered response kinetics lowered not only the temporal resolution of antennal responses to intermittent odor exposure, but also behavioral temporal resolution that turned out to be required for efficient odor source localization. Our results suggest the possibility that Obp83a and Obp83b knockout flies also have lower temporal resolution to intermittent odor stimulation and exhibit behavioral deficits in odor source localization.
We found that the stimuli frequencies to which EAG responses could recover from peak amplitude to baseline levels corresponded to those where behavioral sequences of male moths were reset. Therefore, after peak neuronal activity, returning to baseline activity can be important for tracking odor filaments by continuously resetting behavioral sequences, rather than only peak amplitude detection. Previous studies have reported the temporal sensory resolution of several moth species based on the peak frequency analyses of EAG recordings (Bau et al., 2002, 2005). According to these reports, antennae of male B. mori have a potential temporal resolution to intermittent odor pulses of up to 25 Hz. However, such frequencies are not resolved by the nerve impulse response (Rumbo and Kaissling, 1989; Marion-Poll and Tobin, 1992). Thus, the potential temporal resolution based on peak detection of the EAG response may differ from the temporal sensory resolution for a behavioral reset.
Previous kinetic model analyses have proposed that the inactivation of pheromone molecules following activation of pheromone receptors plays a critical role in controlling the kinetics of pheromone responses and that PBPs are one candidate molecular component involved in pheromone inactivation (Kaissling, 2001, 2009, 2013). In addition to PBPs, several extracellular proteins have been thought to contribute to the sensitivity, selectivity, and inactivation of pheromone detection. For example, the pheromone-degrading enzyme ApolPDE, first identified in A. polyphemus (Ishida and Leal, 2005), exhibits rapid degradation kinetics in vitro that corresponds to the kinetics in antennae, making it an additional candidate to account for the rapid inactivation of sex pheromones in vivo. Although enzymatic degradation of bombykol has been reported in B. mori (Kasang, 1971; Kasang and Kaissling, 1972; Kasang et al., 1989), PDE has not been molecularly identified in this species; its properties have not been tested in vivo or in vitro. To fully understand the molecular mechanisms of pheromone inactivation, future research should look into describing the functions of PDE and how PBPs and PDEs cooperate to achieve rapid inactivation of pheromone signals.
PBPs have been shown to be involved in the sensitive detection of pheromones by efficiently solubilizing pheromone molecules into the sensillum lymph and protecting them from enzymatic degradation during transport to the vicinity of the dendritic membrane of ORNs, where pheromone receptors reside (Ziegelberger, 1995; Shiota et al., 2018). Therefore, PBPs appear to play multiple functional roles both before and after pheromone detection by pheromone receptors. Further electrophysiological, biochemical, and molecular analyses of BmPBP1-knockout moths will help to reveal the detailed mode of action allowing PBPs to perform these different functions during pheromone detection.
In this study, we reported that BmPBP1 contributes to the temporal control of antennal responses, and its absence lowered the temporal sensory resolution, critically affecting efficient odor source localization. Beyond the scope of understanding functional olfactory mechanism, our results could provide strategies to represent spatio-temporal airborne odors in the sensory system by quickly terminating signals after detection, and to reset sequential behavioral patterns in response to sensory input, and are thought to be equally effective for the development of artificial odor source searching algorithms.
Limitations of the study
Although EAG signals are thought to be a summed response of electrical activity across the antenna, the exact mechanisms of EAG response generation is still not fully understood. Therefore, even though our EAG results corresponded adequately to SSRs and behavioral results, we cannot completely exclude the possibility that factors other than PBP knockouts are also involved in the modulation of EAG responses in BmPBP1-KO antennae.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Bombykol | Shiota et al. (2018) | NA |
| Bombykal | Shiota et al. (2018) | NA |
| n-hexane | Wako | 110-54-3 |
| Experimental models: Organisms/strains | ||
| B. mori strain pnd w-1 (+M) | Tamura et al. (2000) | N/A, a standard strain for transgenesis |
| B. mori strain white/c | Kiya et al. (2014) | N/A, a standard strain for maintaining transgenic silkmoths |
| Oligonucleotides | ||
| Genotyping primers | Shiota et al. (2018) | NA |
| Software and algorithms | ||
| R | R Core Team | http://www.r-project.org/index.html |
| MATLAB | MathWorks | https://jp.mathworks.com/ |
| Microsoft Excel (a commercial macroprogram, Statcel version 4) | Seiun-sya | https://seiunsya.co.jp/ |
| Spike2 | Cambridge Electronic Design Limited | http://ced.co.uk/products/spkovin |
| Labview | National Instruments | https://www.ni.com |
| p-Clamp | Molecular Devices | https://www.moleculardevices.com/ |
| Other | ||
| Electrode gel | Parker Laboratories | SPECTRA360 |
| Upright microscope | Olympus | BX50WI |
| Amplifier | Nihon Koden | MEZ-8300 |
| NI DAQ | National Instruments | USB-6210 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to Takeshi Sakurai, PhD (ts206448@nodai.ac.jp).
Materials availability
The BmPBP1-knockout lines used in this study will be made available upon request.
Experimental model and subject details
Animals
We used BmPBP1-knockout moths that were generated using transcription activator-like effector nuclease (TALEN)-mediated gene targeting, as described previously (Shiota et al., 2018). Larvae were reared on an artificial diet (Nihon Nosan Kogyo, Yokohama, Japan) at 25°C under a 16:8 h light-dark cycle. Following eclosion, one-to-seven-day-old male moths were used in the experiments. Subsequent to all experiments in this study, the genotypes of all BmPBP1-knockout males were determined by PCR, as previously described (Shiota et al., 2018).
Method details
Chemicals
Bombykol and bombykal were kindly provided by Dr. S. Matsuyama of University of Tsukuba, Japan. The purity (>99.5%) of synthetic bombykol and bombykal was verified by gas chromatography using previously described conditions (Fujii et al., 2010; Uehara et al., 2016).
Electroantennogram (EAG) recordings
An antenna of a male moth was excised at the base, and a few antennomeres at the tip were cut off. The antenna was then mounted on the EAG probe using electrode gel (SPECTRA 360; Parker Laboratories, Fairfield, NJ, USA). A glass cartridge (5 mm inner diameter, 10 cm length) was placed 1 cm from the antennae and prepared for stimulation by inserting a piece of filter paper (1.5 × 1.5 cm), and 5 μL of a pheromone solution in n-hexane was administered. A charcoal-purified airstream (1 L/min) was passed through the glass cartridge and directed onto the antenna. The EAG responses were amplified using a custom-made amplifier (Minegishi and Kanzaki, unpublished), band-pass filtered at 0.16 to 300 Hz, and digitized at 1 kHz (USB-6210; National Instruments, Austin, TX, USA). The data were analyzed using a custom-written program (MATLAB; Mathworks, Natick, MA, USA). Baseline levels were calculated from 2 s of data recording before the stimulus. The start time of the response was defined as the time at which the EAG amplitude first exceeded the baseline level by ± 2.5 standard deviations. The response time constant was defined as the time of the response phase, from the start of response to 63.2% of the absolute EAG response amplitude. The recovery time constant was defined as the time of the settling phase, from the peak amplitude to 63.2% of the absolute EAG response amplitude. For evaluation of the significance of the EAG time constants, to rule out the effect of EAG amplitude on response kinetics, we plotted the time parameters as a function of peak amplitude. Thus, each time parameter of the EAG (recovery time, response time, and latency) could be compared at the same EAG amplitude between moths. The valley amplitude was defined as the EAG response amplitude, where each local minimum amplitude was subtracted from the baseline level. The termination time was defined as the time from the peak amplitude to the baseline level by ± 5% standard error of the mean (SEM). The latency was calculated from the start time by subtracting the arrival time of the pheromone on the antenna that was estimated using a method previously described (Szyszka et al., 2014).
Single sensillum recording
Single sensillum recordings (SSRs) were performed in a Faraday cage. Moths were fixed on a custom-made acrylic chamber under an Olympus BX50WI (500×) microscope. The antennae were stabilized with dental wax (GC Corporation, soft plate wax), and the basement segments were fixed with a resin bond (GC Corporation, G-Fix). Spike responses were recorded by a sharpened tungsten wire electrode (diameter 0.5 mm, tip approximately 1 μm) inserted into the bases of pheromone sensitive long sensilla trichodea on the antennae. As a reference electrode, a silver wire was inserted into the compound eye of the moth. Pheromone was delivered by injecting pheromone pulses into a charcoal-purified and moistened continuous airstream (1 L/min), to reduce any mechanical artifacts. Spike responses were band-pass filtered at 300 to 3 kHz and amplified (Nihon Koden, MEZ-8300). All spike responses were digitized at 10 kHz. Normalized response is calculated from spike number divided by max spike number in each time bin. The spontaneous spike rate was calculated from 2 s of data recording before the stimulus, with a time bin of 100 ms. The termination time of spike responses was defined as the time required to return to the spontaneous spike level after 1st simulation. We analyzed data with a custom MATLAB (Mathworks, MA, Natick, USA) program and Spike2 software (Cambridge Electronic Design Limited, UK).
Behavioral experiments using tethered moths
Scales on the dorsal thorax of moths were removed, and a copper wire holder was attached to the thorax with glue. Moths were placed on the top of a 70 mm diameter polystyrene foam ball (track ball). The ball was supported by a plastic funnel with an electrical fan attached under the ball. The air flow generated by the electric fan kept the ball floating in the air to reduce friction and enable moths to behave during locomotion. Pulsed pheromone stimulation of 200 ms duration at 0.83 Hz and 2 Hz frequency and stimulation of 1 s at 0.17 Hz frequency was used. Wild-type and BmPBP1-knockout males were stimulated with 100 ng and 10,000 ng bombykol, respectively. Based on a previous study using tethered moths, the surge behavior (Figure S2) was defined as straight walking with a small angle, at an accumulated angular velocity ≤8°/s, when the accumulated walking distance was ≥1 mm, with a forward velocity ≥1 mm/s, using a time bin of 200 ms. The zigzag turn behavior was defined as turning behavior with a large angle, at an accumulated angular velocity ≥8°/s, with an accumulated forward velocity ≤10 mm/s. These parameters were adjusted to remove oscillations caused by the moth’s movements on the track ball according to a previous study (Pansopha et al., 2014). Pheromone source searching behaviors of moths were recorded with an optical mouse attached behind the polystyrene ball. Surge rate was defined as the ratio of total surge duration to total locomotion duration containing both surge and zigzag turns. We processed data based on these criteria with a custom MATLAB (Mathworks, MA, Natick, USA) program.
Wind tunnel experiments
We performed odor source localization experiments in a wind tunnel (1800 × 900 × 300 mm L × W × H) at 25–28°C. Pulsed pheromone stimulation of 200 ms duration at 2 Hz frequency was controlled by an electric valve, and wind velocity was controlled at 0.5–0.7 m/s. Air is continuously exhausted by air pump in the downstream toward odor source to make stable air flow. For the odor source localization experiments, both BmPBP1-knockout and wild-type male moths were used within 1 to 4 days after eclosion. Synthetic bombykol (1, 10, 100, 1000 and 10,000 ng) and wild-type females were used for pheromone stimulation. Moths were placed 50 cm downstream of a pheromone source. The pheromone source searching behavior of male moths was captured with a digital video camera at a frame rate of 30 Hz. Surge was defined as straight walking with a small angle, at an angular velocity ≤5°/s, with the accumulated turn angle ≤30°/s, with a forward velocity ≥1 mm/s and a walking distance ≥1 mm, using a bin size of 200 ms based on previous studies (Ando and Kanzaki, 2015). All turn angles were defined as the total cumulative turn angle during pheromone searching behavior. Behaviors that did not correspond to these criteria were seen as stopping and switching directions and were not included in our analyses. The other behaviors (at angular velocity ≥5°/s, with accumulated turn angle ≥30°/s) were classified as zigzag turns. The success rate was calculated as the number of moths that successfully localized the pheromone source divided by the total number of moths tested. We processed image data based on these criteria with customized Java and R scripts (R Development Core Team).
Quantification and statistical analysis
Statistical analysis
In our experiments, to assess the statistical differences between wild-type and BmPBP1-knockout moths, we used Student’s t-test, Fisher’s exact probability test, the Steel–Dwass test and an ANCOVA test. Statistical significance was calculated using Microsoft Excel 2016, a commercial macroprogram (Statcel version 4; Seiun-sya, Japan) and custom VBA and MATLAB (Mathworks, MA, Natick, USA) programs. The error bars shown in the figures represent the SEMs. The asterisks indicate significant differences between the groups (∗p < 0.05, ∗∗p < 0.01). The different letters indicate significant differences according to the Steel–Dwass test and Fisher’s exact probability test.
Acknowledgments
We thank Dr. Ryo Minegishi for the development of the EAG amplifier, Mr. Takuya Nakajo and Ms. Junko Tsuchiya and Ms. Akane Kitazono-Itoigawa for technical assistance in rearing silkmoths, and Dr. Shigeru Matsuyama for providing bombykol. This work was supported by a Grant-in-Aid for Young scientists (A) (26712027), Grant-in-Aid for Scientific Research (B) (18H02211) from Japan Society for the Promotion of Science (JSPS), Japan, awarded to T.S. and by a Grant-in-Aid for Scientific Research (B) (15H04399) from JSPS, Japan, awarded to R.K.
Author contributions
Y.S., T.S., and R.K. designed the research; Y.S., T.S., N.A., S.S.H., and H.M. performed the research; T.D. contributed new reagents/analytic tools; Y.S. and T.S. analyzed the data; and Y.S. and T.S. wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: November 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103334.
Supplemental information
Data and code availability
Section 1: All data reported in this paper will be shared by the lead contact upon request.
Section 2: This paper does not report original code.
Section 3: Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Ando N., Kanzaki R. A simple behavior provides accuracy and flexibility in odor plume tracking-the robotic control of sensory-motor coupling in silkmoths. J. Exp. Biol. 2015;218:3845–3854. doi: 10.1242/jeb.124834. [DOI] [PubMed] [Google Scholar]
- Baker T.C., Willis M.A., Haynes K.F., Phelan P.L. A pulsed cloud of sex pheromone elicits upwind flight in male moths. Physiol. Entomol. 1985;10:257–265. [Google Scholar]
- Baker T.C., Hansson B.S., Löfstedt C., Löfqvist J. Adaptation of antennal neurons in moths is associated with cessation of pheromone-mediated upwind flight. Proc. Natl. Acad. Sci. U S A. 1988;85:9826–9830. doi: 10.1073/pnas.85.24.9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T.C. In: ISOT X, Proc. 10th Intl. Symp. Olfac. Taste. Døving K., editor. GCS/AS; 1990. Upwind flight and casting flight: complementary phasic and tonic systems used for location of sex pheromone sources by male moths; pp. 18–25. [Google Scholar]
- Bau J., Justus K.A., Cardé R.T. Antennal resolution of pulsed pheromone plumes in three moth species. J. Insect Physiol. 2002;48:433–442. doi: 10.1016/s0022-1910(02)00062-8. [DOI] [PubMed] [Google Scholar]
- Bau J., Justus K.A., Loudon C., Cardé R.T. Electroantennographic resolution of pulsed pheromone plumes in two species of moths with bipectinate antennae. Chem. Senses. 2005;30:771–780. doi: 10.1093/chemse/bji069. [DOI] [PubMed] [Google Scholar]
- Butenandt A., Beckmann R., Stamm D., Hecker E. Über den Sexual-Lockstoff des Seidenspinners Bombyx mori. Reindarstellung und Konstitution. Z. Naturforsch. B. 1959;14:283–284. [Google Scholar]
- Forstner M., Gohl T., Breer H., Krieger J. Candidate pheromone binding proteins of the silkmoth Bombyx mori. Invert. Neurosci. 2006;6:177–187. doi: 10.1007/s10158-006-0032-0. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Kazawa T., Sakurai T., Fukushima R., Uchino K., Yamagata T., Namiki S., Haupt S.S., Kanzaki R. Odorant concentration differentiator for intermittent olfactory signals. J. Neurosci. 2014;34:16581–16593. doi: 10.1523/JNEUROSCI.2319-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Nakano R., Takubo Y., Qian S., Yamakawa R., Ando T., Ishikawa Y. Female sex pheromone of a lichen moth Eilema japonica (Arctiidae, Lithosiinae): components and control of production. J. Insect Physiol. 2010;56:1986–1991. doi: 10.1016/j.jinsphys.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Gräter F., Xu W., Leal W., Grubmueller H. Pheromone discrimination by the pheromone-binding protein of Bombyx mori. Structure. 2006;14:1577–1586. doi: 10.1016/j.str.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Große-Wilde E., Svatos A., Krieger J. A pheromone-binding protein mediates the bombykol-induced activation of a pheromone receptor in vitro. Chem. Senses. 2006;31:547–555. doi: 10.1093/chemse/bjj059. [DOI] [PubMed] [Google Scholar]
- Haupt S.S., Sakurai T., Maniki S., Kazawa T., Kanzaki R. In: The Neurobiology of Olfaction. Menini A., editor. CRC Press; 2010. Olfactory information processing in moths; pp. 71–112. [Google Scholar]
- Hooper A.M., Dufour S., He X., Muck A., Zhou J.-J., Almeida R., Field L.M., Svatos A., Pickett J.A. High-throughput ESI-MS analysis of binding between the Bombyx mori pheromone-binding protein BmorPBP1, its pheromone components and some analogues. Chem. Commun. 2009;14:5725–5727. doi: 10.1039/b914294k. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Leal W.S. Rapid inactivation of a moth pheromone. Proc. Natl. Acad. Sci. U S A. 2005;102:14075–14079. doi: 10.1073/pnas.0505340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaissling K.E. Olfactory perireceptor and receptor events in moths: a kinetic model. Chem. Senses. 2001;26:125–150. doi: 10.1093/chemse/26.2.125. [DOI] [PubMed] [Google Scholar]
- Kaissling K.E. Olfactory perireceptor and receptor events in moths: a kinetic model revised. J. Comp. Physiol. A Neurothol. Sens. Neural Behav. Physiol. 2009;195:895–922. doi: 10.1007/s00359-009-0461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaissling K.E., Kasang G., Bestmann H.J., Stransky W., Vostrowsky O. A new pheromone of the silkworm moth Bombyx mori. Sensory pathway and behavioral effect. Naturwissenschaften. 1978;65:382–384. [Google Scholar]
- Kaissling K.E. Kinetics of olfactory responses might largely depend on the odorant – receptor interaction and the odorant deactivation postulated for flux detectors. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2013;199:879–896. doi: 10.1007/s00359-013-0812-z. [DOI] [PubMed] [Google Scholar]
- Kanzaki R., Sugi N., Shibuya T. Self-generated zigzag turning of Bombyx mori males during pheromone-mediated upwind walking. Zool. Sci. 1992;9:515–527. [Google Scholar]
- Kasang G. In: Gustation and Olfaction. Ohloff G., Thomas A.F., editors. Academic Press; 1971. Bombykol reception and metabolism on the antennae of the silkmoth Bombyx mori; pp. 245–250. [Google Scholar]
- Kasang G., Kaissling K.E. In: Intern. Symp. Olfaction and Taste IV. Schneider D., editor. Wissensch Verlagsgesellsch Stuttgart; 1972. Specificity of primary and secondary olfactory processes in Bombyx antennae; pp. 200–206. [Google Scholar]
- Kasang G., Nicholls M., von Proff L. Sex pheromone conversion and degradation in antennae of the male silkworm moth Bombyx mori L. Experientia. 1989;45:81–87. [Google Scholar]
- Kennedy J.S., Ludlow A.R., Sanders C.J. Guidance system used in moth sex attraction. Nature. 1980;288:475–477. [Google Scholar]
- Kennedy J.S., Ludlow A.R., Sanders C.J. Guidance of flying male moths by wind borne sex pheromone. Physiol. Entomol. 1981;6:395–412. [Google Scholar]
- Kiya T., Morishita K., Uchino K., Iwami M., Sezutsu H. Establishment of tools for neurogenetic analysis of sexual behavior in the silkmoth, Bombyx mori. PLoS One. 2014;9:e113156. doi: 10.1371/journal.pone.0113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer E. In: Mechanisms in Insect Olfaction. Payne T.L., Birch M.C., Kennedy C.E.J., editors. Oxford University Press; 1986. Turbulent diffusion and pheromone-triggered anemotaxis; pp. 59–67. [Google Scholar]
- Kramer E. Attractivity of pheromone surpassed by time-patterned application of two nonpheromone compounds. J. Insect Behav. 1992;5:83–97. [Google Scholar]
- Linn C.E., Campbell M.G., Roelofs W.L. Temperature modulation of behavioral thresholds controlling male moth sex pheromone response specificity. Physiol. Entomol. 1988;12:291–306. [Google Scholar]
- Maida R., Mameli M., Müller B., Krieger J., Steinbrecht R.A. The expression pattern of four odorant-binding proteins in male and female silk moths, Bombyx mori. J. Neurocytol. 2005;34:149–163. doi: 10.1007/s11068-005-5054-8. [DOI] [PubMed] [Google Scholar]
- Marion-Poll F., Tobin T.R. Temporal coding of pheromone pulses and trains in Manduca sexta. J. Comp. Physiol. A Neurothol. Sens. Neural Behav. Physiol. 1992;171:505–512. doi: 10.1007/BF00194583. [DOI] [PubMed] [Google Scholar]
- Murlis J., Jones C. Fine-scale structure of odor plumes in relation to insect orientation to distant pheromone and other attractant sources. Physiol. Entomol. 1981;6:71–86. [Google Scholar]
- Murlis J., Elkinton J.S., Cardé R.T. Odor plumes and how insects use them. Annu. Rev. Entomol. 1992;37:505–532. [Google Scholar]
- Murlis J., Willis M.A., Cardé R.T. Spatial and temporal structures of pheromone plumes in fields and forests. Physiol. Entomol. 2000;25:211–222. [Google Scholar]
- Pansopha P., Ando N., Kanzaki R. Dynamic use of optic flow during pheromone tracking by the male silkmoth, Bombyx mori. J. Exp. Biol. 2014;217:1811–1820. doi: 10.1242/jeb.090266. [DOI] [PubMed] [Google Scholar]
- Rumbo E.R., Kaissling K.E. Temporal resolution of odor pulses by three types of pheromone receptor cells in Antheraea polyphemus. J. Comp. Physiol. A Neurothol. Sens. Neural Behav. Physiol. 1989;165:281–291. [Google Scholar]
- Sakurai T., Nakagawa T., Mitsuno H., Mori H., Endo Y., Tanoue S., Yasukochi Y., Touhara K., Nishioka T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc. Natl. Acad. Sci. U S A. 2004;101:16653–16658. doi: 10.1073/pnas.0407596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., Namiki S., Kanzaki R. Molecular and neural mechanisms of sex pheromone reception and processing in the silkmoth Bombyx mori. Front. Physiol. 2014;5:125. doi: 10.3389/fphys.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermann E.A., Smith D.P. Odor-specific deactivation defects in a drosophila odorant-binding protein mutant. Genetics. 2019;213:897–909. doi: 10.1534/genetics.119.302629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota Y., Sakurai T., Daimon T., Mitsuno H., Fujii T., Matsuyama S., Sezutsu H., Ishikawa Y., Kanzaki R. In vivo functional characterisation of pheromone binding protein-1 in the silkmoth, Bombyx mori. Sci. Rep. 2018;8:13529. doi: 10.1038/s41598-018-31978-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszka P., Gerkin R.C., Galizia C.G., Smith B.H. High-speed odor transduction and pulse tracking by insect olfactory receptor neurons. Proc. Natl. Acad. Sci. U S A. 2014;111:16925–16930. doi: 10.1073/pnas.1412051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Thibert C., Royer C., Kanda T., Abraham E., Kamba M., Komoto N., Thomas J.L., Mauchamp B. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat. Biotechnol. 2000;18:81–84. doi: 10.1038/71978. [DOI] [PubMed] [Google Scholar]
- Uehara T., Kitahara H., Naka H., Matsuyama S., Ando T., Honda H. Single-component pheromone consisting of bombykal in a diurnal hawk moth, Neogurelca himachala sangaica. J. Chem. Ecol. 2016;42:517–522. doi: 10.1007/s10886-016-0714-y. [DOI] [PubMed] [Google Scholar]
- Vickers N.J., Baker T.C. Reiterative responses to single strands of odor promote sustained upwind flight and odor source location by moths. Proc. Natl. Acad. Sci. U S A. 1994;91:5756–5760. doi: 10.1073/pnas.91.13.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt R.G., Riddiford L.M. Pheromone binding and inactivation by moth antennae. Nature. 1981;293:161–163. doi: 10.1038/293161a0. [DOI] [PubMed] [Google Scholar]
- Vogt R.G., Große-Wilde E., Zhou J.J. The Lepidoptera odorant binding protein gene family: gene gain and loss within the GOBP/PBP complex of moths and butterflies. Insect Biochem. Mol. Biol. 2015;62:142–153. doi: 10.1016/j.ibmb.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Ye Z.F., Liu X.-L., Han Q., Lioa H., Dong X.-T., Zhu G.-H., Dong S.-L. Functional characterization of PBP1 gene in Helicoverpa armigera (Lepidoptera: Noctuidae) by using the CRISPR/Cas9 system. Sci. Rep. 2017;7:8470. doi: 10.1038/s41598-017-08769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelberger G. Redox-shift of the pheromone-binding protein in the silkmoth Antheraea polyphemus. Eur. J. Biochem. 1995;232:706–711. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Section 1: All data reported in this paper will be shared by the lead contact upon request.
Section 2: This paper does not report original code.
Section 3: Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.