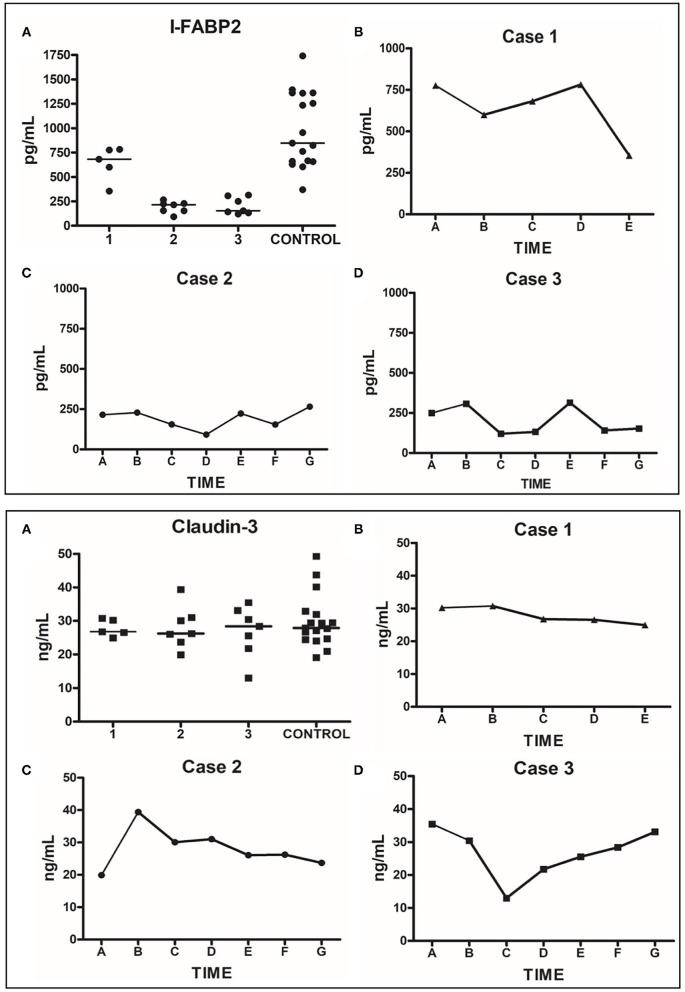
Figure 4.
Human plasma I-FABP2 and Claudin-3 analysis. Top Panel: I-FABP2. (A) Grouped values for each patient during their hospitalization compared to grouped values of controls. (B) Daily sequential values for Case 1; time point A represents day 7 of hospitalization (day 3 of anakinra), when patient was clinically stable with resolution of diarrhea; the subsequent points are from daily samples obtained over the next 5 days until the day of discharge. (C) Daily sequential values for Case 2; time point A represents day 2 of hospitalization (day prior to onset of diarrhea, day after the appendectomy); the subsequent points are from daily samples obtained over the next 7 days when the diarrhea developed— requiring treatment with anakinra and steroids leading to resolution of symptoms; the last sample was from the day of discharge. (D) Daily sequential values for Case 3; time point A represents the day 2 of hospitalization when the patient was diagnosed with MIS-C and received the first dose of anakinra and steroids. The subsequent points are from daily samples obtained over the next week of his hospitalization, with the last sample obtained on the day of discharge. Bottom Panel: Claudin-3. (A–D) described the same as Top Panel (A–D), respectively.