Extended Data Fig. 5 |. β-hairpin extension and prepore-to-pore transition.
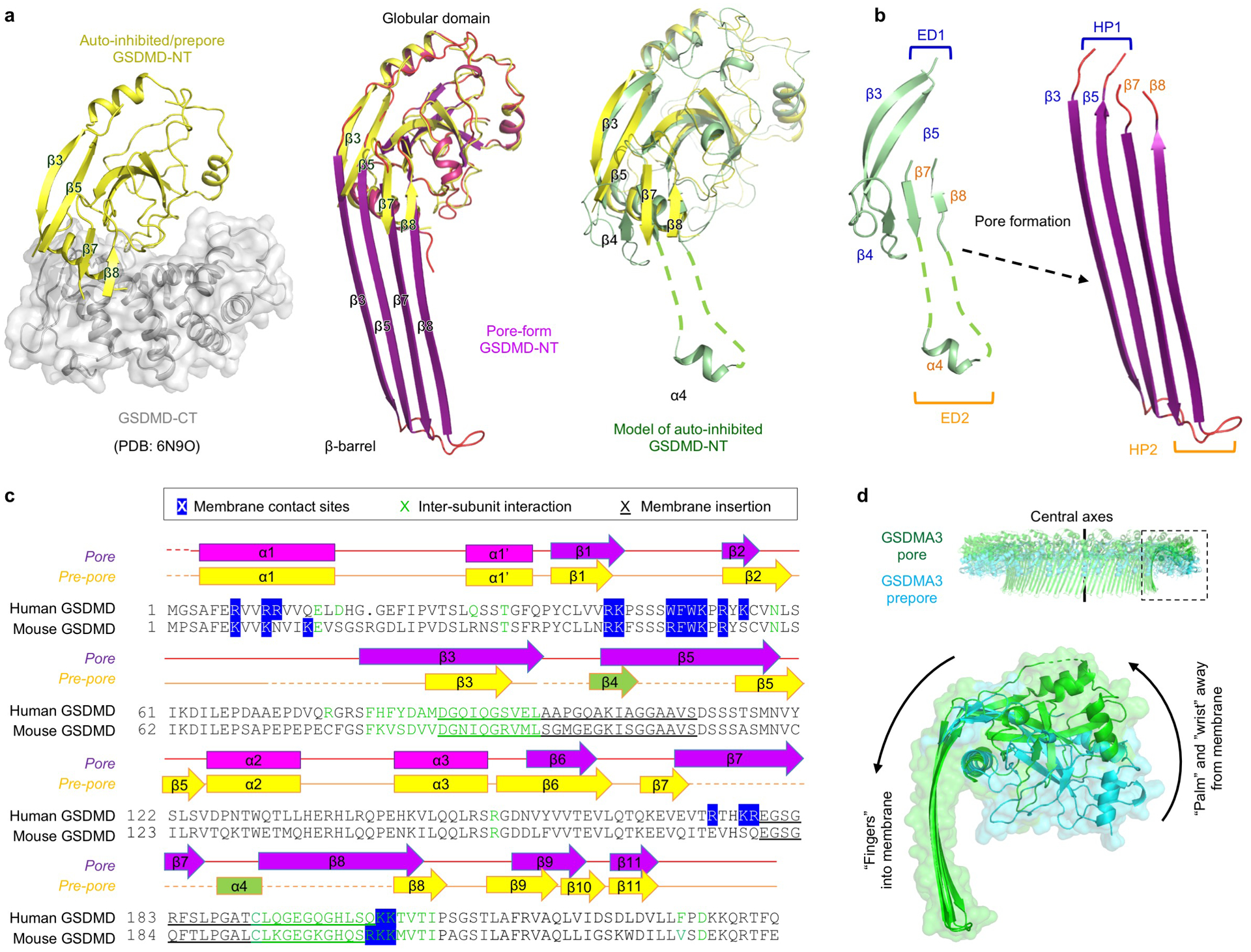
a, Comparison between auto-inhibited, prepore-form, and pore-form GSDMD. The auto-inhibited GSDMD-NT was obtained from the crystal structure of full-length GSDMD (PDB: 6N9O). The β4 strand and α4 helix are invisible in the crystal structure and were modelled based on the crystal structure of full-length GSDMA3 (PDB: 5B5R). b, Formation of β-hairpins (HPs). The β3-β4-β5 region constitute the first extension domain (ED1), which transforms into HP1 by refolding. The β7-α4-β8 region represents ED2 and becomes HP2. c, Sequence alignment of human and mouse GSDMD with secondary structures and key residues denoted. Blue highlight: Responsible for lipid binding, through either hydrophobic or charged interactions. Green: At inter-subunit interfaces. Underscore: Important for membrane insertion. d, Conserved rigid-body movement of the globular domain (“palm”) towards the membrane-distal direction during GSDM pore formation, shown by alignment of the GSDMA3 pore structure (PDB: 6CB8) and prepore model at their central axes.
