Figure 4. The unfolded protein response (UPR) and ER stress are at the crossroads of neuronal cell survival and cell death.
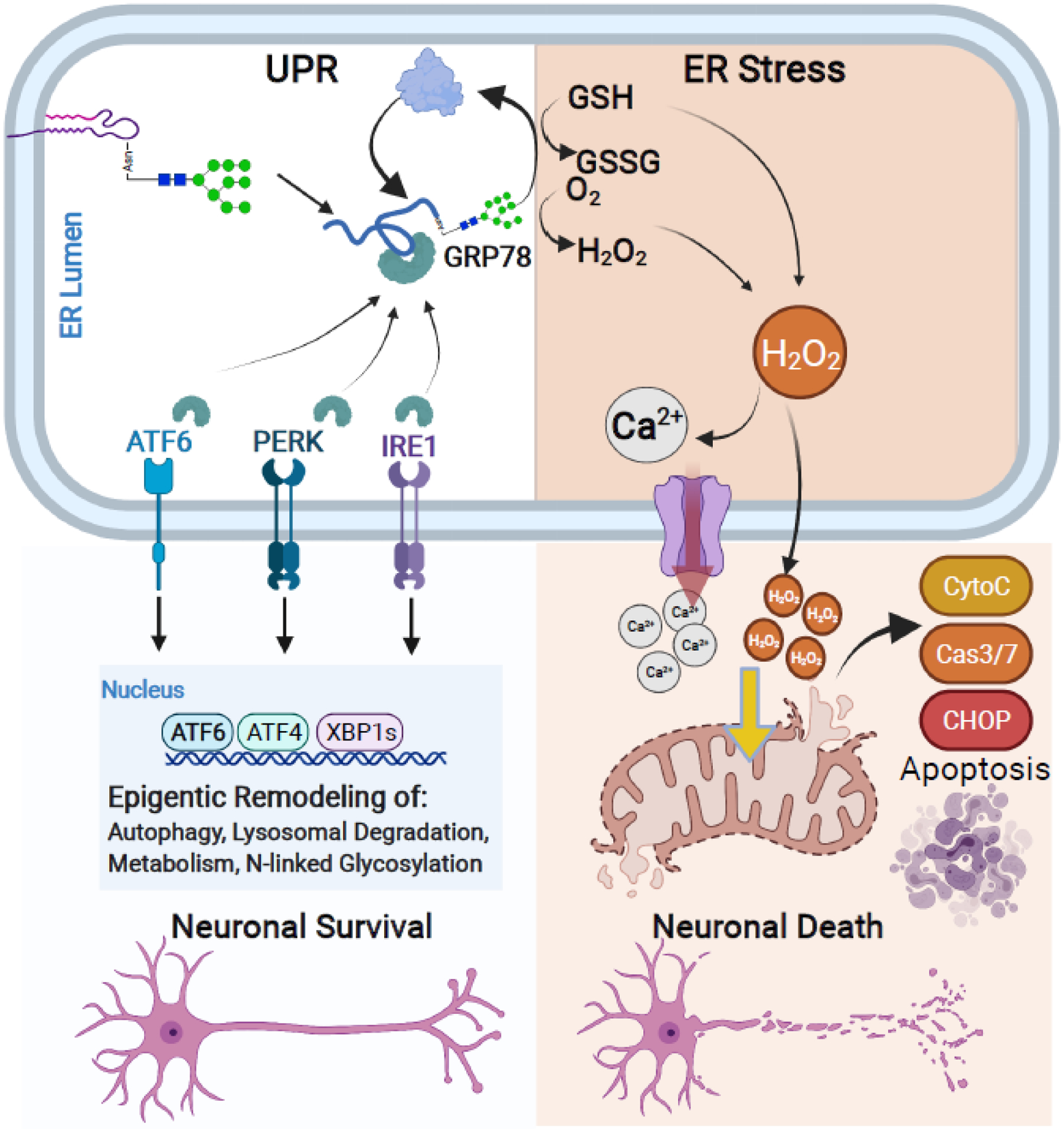
Early N-linked glycan biosynthesis is a central mediator of the UPR pathway. The UPR leads to protein refolding or salvage and activation of pro-survival pathways in neurons. However, if accumulation of unfolded proteins or prolonged stress exceeds capacity, the UPR leads to ER stress that triggers mitochondrial apoptotic signaling cascades and neuronal cell death. The UPR and pro-survival pathways (Left): Accumulation of misfolded proteins recruits available GRP78 and sequesters GRP78 away from ER membrane signaling transducers: ATF6, PERK, and IRE1. Binding of GRP78 to misfolded glycoproteins leads to protein refolding or salvage through lysosomal and proteasomal degradation. Dissociation of GRP78 with ATF6, PERK, and IRE1 also leads to their activation and subsequent signaling cascades for epigenetic remodeling of neurons. This process promotes neuronal cell survival by upregulating genes involved in the autophagy, lysosomal processing, metabolism, and N-linked glycosylation as a protective process against cellular stress. ER stress occurs when misfolded proteins or stress exceeds the UPR capacity (Right): If the UPR fails to re-establish cellular homeostasis, excess utilization and depletion of GSH and production of H2O2 during the refolding process leads to the ectopic release of Ca2+ and H2O2 from the ER into the cytosol. Excess of either cytosolic Ca2+ and H2O2 initiates mitochondrial-driven pro-apoptotic pathways including release of Cytochrome C and Caspase 3/7 activation and signaling cascade, ultimately inducing neuronal cell death.
