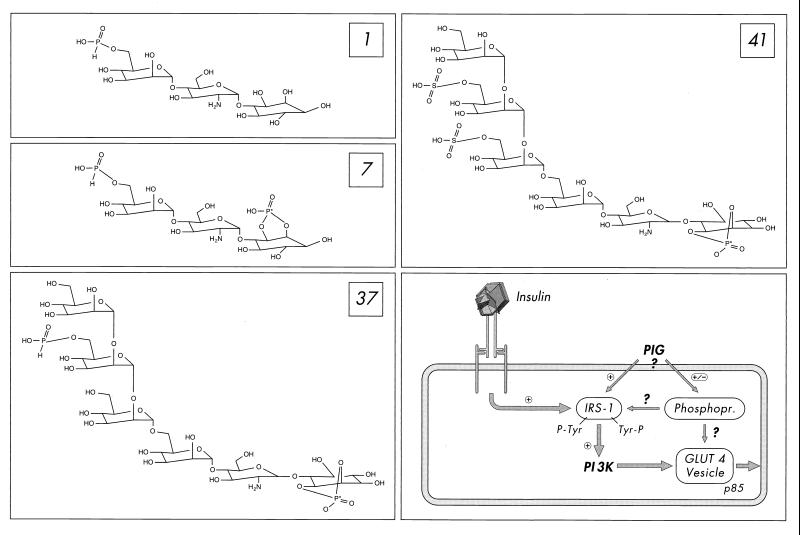
FIG. 1.
Structure of the PIG compounds 1, 7, 37, and 41 (with the activity-determining phosphate moiety marked by an asterisk) and their mode of action in fat and muscle cells according to references 12 and 22 (also see the introduction). PIG compounds induce tyrosine phosphorylation of IRS proteins and their association with PI 3K, which is thereby activated, as does insulin by stimulating the insulin receptor tyrosine kinase. Operation of the IRS-1–PI 3K pathway (and possibly membrane association of the regulatory p85 subunit of PI 3K) is a prerequisite for fusion of GLUT4-containing vesicles with the plasma membrane, the so-called GLUT4 translocation, but may not be sufficient (also see Discussion). In addition, PIG compounds modulate the phosphorylation state of a number of phosphoproteins, which are not affected by insulin (22), but may be required for PIG-dependent GLUT4 translocation.