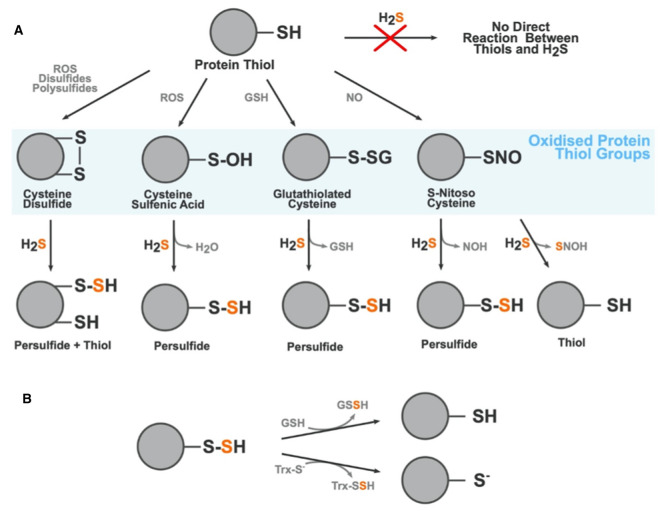
Figure 2. Formation of protein persulfides by H2S.
(A) Modification of cysteine residues by H2S. H2S cannot directly modify thiol groups (i.e. cysteine residues). The thiol group must first be must first be oxidised into a disulfide (disulfide bond formation), sulfenic acid (S-Sulfenylation), glutathiolated cysteine (S-Glutathiolation), or a S-Nitroso Cysteine (S-Nitrosation). From these oxidised thiol groups H2S can react to form persilfides, thiols, and a variety of by-products dependent on the type of oxidised thiol it is reacting with. The sulfur atom from the H2S molecule is highlighted in orange to show where in the product it incorporates. (B) Persulfidation is a reversible post-translational modification and can be readily removed by the action of glutathione and thioredoxin. ROS, Reactive oxygen species; GSH, Glutathione; NO, Nitric oxide; NOH, Nitroxyl; SNOH, Thionitrous acid; GSSH, Glutathione persulfide; Trx-S−, Thioredoxin.