Abstract
Attenuating β amyloid- (Aβ-) induced microglial activation is considered to be effective in treating Alzheimer's disease (AD). Berberine (BBR) can reduce microglial activation in Aβ-treated microglial cells; the mechanism, however, is still illusive. Silencing of cytokine signaling factor 1 (SOCS1) is the primary regulator of many cytokines involved in immune reactions, whose upregulation can reverse the activation of microglial cells. Microglia could be activated into two different statuses, classic activated state (M1 state) and alternative activated state (M2 state), and M1 state is harmful, but M2 is beneficial. In the present study, N9 microglial cells were exposed to Aβ to imitate microglial activation in AD. And Western blot and immunocytochemistry were taken to observe inducible nitric oxide synthase (iNOS), Arginase-1 (Arg-1), and SOCS1 expressions, and the enzyme-linked immunosorbent assay (ELISA) was used to measure inflammatory and neurotrophic factor release. Compared with the normal cultured control cells, Aβ exposure markedly increased the level of microglial M1 state markers (P < 0.05), including iNOS protein expression, tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and IL-6 releases, and BBR administration upregulated SOSC1 expression and the level of microglial M2 state markers (P < 0.05), such as Arg-1 expression, brain-derived neurotrophic factor (BDNF), and glial cell-derived neurotrophic factor (GDNF) releases, downregulating the SOCS1 expression by using siRNA, however, significantly reversed the BBR-induced effects on microglial M1 and M2 state markers and SOCS1 expression (P < 0.05). These findings indicated that BBR can inhibit Aβ-induced microglial activation via modulating the microglial M1/M2 activated state, and SOCS1 mediates the process.
1. Introduction
Alzheimer's disease (AD) is one of the most common neurodegenerative disorders, characterized as progressive memory and cognitive dysfunctions, leading to great economic and social burden to the family with AD patients [1–3]. However, unfortunately, there is very limited medicine has been proved to be effective for AD. Therefore, searching for effective methods or therapy for AD is very urgent. Many studies reported that accumulation of β amyloid (Aβ) in brain tissue is the key pathological character in the development of AD [4, 5]. And a high level of Aβ in the brain can activate microglial cells and induce neuroinflammation [6, 7]. Additionally, chronic inflammation in brain tissue can result in neuronal injury and even death [8, 9]. For this reason, alleviating Aβ-induced microglial activation is regarded as an effective therapy for AD. Activated microglial cells could be divided into two statuses, classic activated state (M1 state) and alternative activated state (M2 state) [10–12]. As the microglial cells of M1 state can secrete proinflammatory cytokines, including tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and IL-6, the M1 state is believed to be harmful to the brain; in contrast, the microglia of M2 state can release anti-inflammatory and neurotrophic factors, such as IL-10, brain-derived neurotrophic factor (BDNF), and glial cell-derived neurotrophic factor (GDNF), which is considered to be beneficial [13]. Silencing of cytokine signaling factor 1 (SOCS1) is the primary regulator of many cytokines, mainly expressed in immunocytes, including macrophages in the peripheral tissue and microglial cells of the central nervous system (CNS). And many investigations showed that SOCS1 upregulation could reduce microglial activation and neuroinflammation [14, 15].
Berberine (BBR) is a bioactive substance from Coptis chinensis, a traditional Asian herbal medicine [16]. Traditionally, BBR is used to treat bacterial gastroenteritis, and recently, an increasing number of studies indicated that BBR can alleviate Aβ-induced neurotoxicity in vivo and in vitro [17, 18]. These evidences above showed that BBR could be effective in treating AD; the mechanism, however, is still obscure.
In the present study, we used Aβ to activate N9 microglial cells to mimic neuroinflammation of AD and investigated the role of SOCS1 in the BBR-induced anti-inflammatory effects and the potential neuroprotection of BBR.
2. Materials and Methods
2.1. Cells and Reagents
The N9 microglial cells were obtained from the Air Force Medical University. Berberine, β amyloid 1-42 peptide (Aβ), Iscove's modified Dulbecco's medium (IMDM), fetal bovine serum (FBS), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide (MTT), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The enzyme-linked immunosorbent assay (ELISA) reagent kits, including TNF-α, IL-1β, BDNF, GDNF, and IL-10, were purchased from PeproTech Inc. (USA). The anti-iNOS (ab115819), anti-Arginase-1 (anti-Arg-1, ab124917), and anti-SOCS1 (ab9870) primary antibodies were obtained from Abcam (Cambridge, UK). The Cy-3-labeled secondary antibody, FITC-labeled secondary antibody, and anti-β-actin, anti-GAPDH, and secondary horseradish peroxidase-conjugated goat anti-rabbit primary antibody were obtained from Beijing Bioscience Co. Ltd. (Beijing, China). The 4′,6-diamidino-2-phenylindole (DAPI) staining solution was purchased from Beyotime (China).
2.2. Cell Culture and Treatments
The N9 microglial cells were cultured in the IMDM medium, containing 5% FBS, 1% (v : v) penicillin/streptomycin solution (Solarbio Life Sciences, Beijing, China), and 1 mM glutamine. The humidity of the incubator was 100%, the temperature was 37°C, the atmosphere of the incubator consisted of 95% O2 and 5% CO2, and the medium was changed every 2-3 days. The cells were passaged 2-3 times per week and used within 10 weeks.
To find a suitable BBR treatment concentration, the cells were divided into five groups (Figure 1(a)), including the normal cultured control group, 5 μM Aβ exposure group (Aβ), and three BBR treatment groups (cells were exposed to the medium containing 0.1 μM, 1 μM, or 5 μM BBR plus 5 μM Aβ); after 24 h incubation, iNOS expression and TNF-α concentration were measured. To observe BBR-induced effects on proinflammatory factor releases, the cells were divided into four groups (Figure 1(b)), including control, 5 μM Aβ exposure group (Aβ), 5 μM BBR+5 μM Aβ treatment group (BBR+Aβ), and 5 μM BBR treatment group (BBR); after 24 h incubation, IL-1β and IL-6 concentrations in the medium were measured. To study the role of SOCS1 in the BBR-induced inhibition of microglial activation (Figure 1(c)), the cells were divided into five groups, including control, 5 μM Aβ exposure group (Aβ), 5 μM BBR+5 μM Aβ treatment group (BBR+Aβ), SOCS1-siRNA treatment group (SOCS-siRNA+BBR+Aβ), and scrambled- (SC-) siRNA treatment group (SC-siRNA+BBR+Aβ); after the treatments, microglial activation markers were evaluated to assess the activation degree of the cells.
Figure 1.
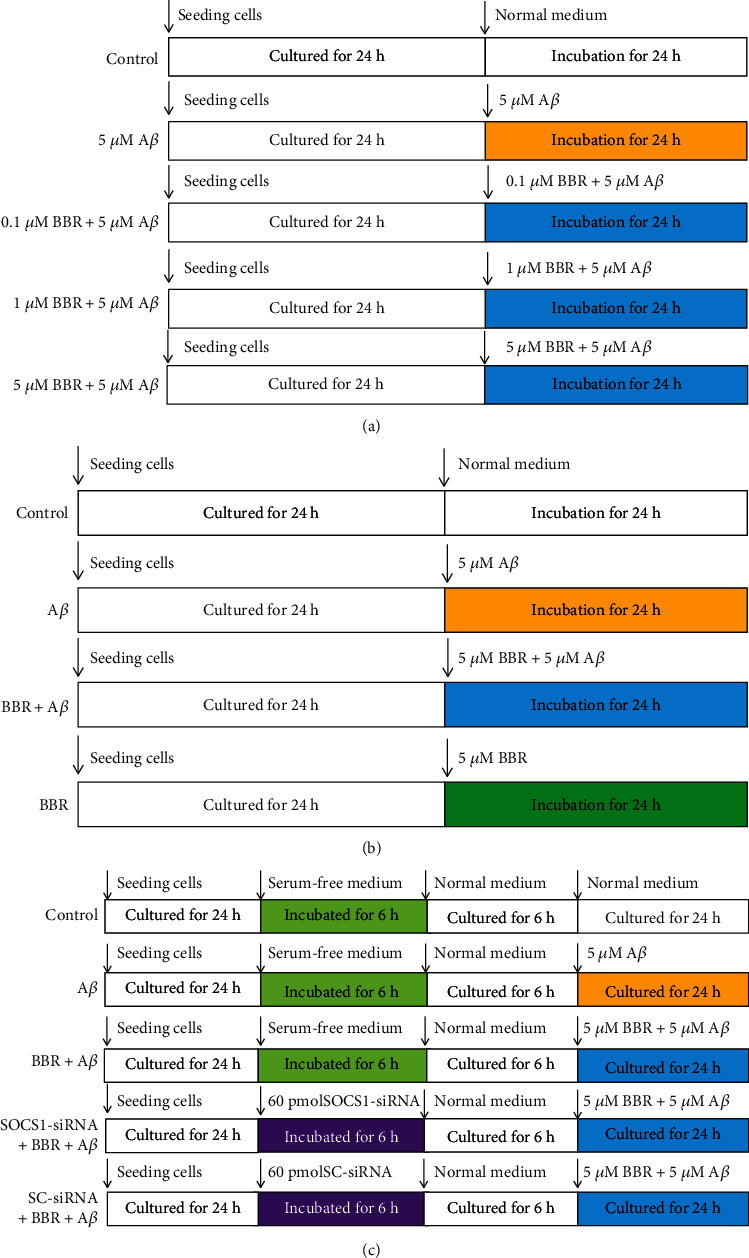
Experimental protocols. (a) Finding a suitable berberine (BBR) concentration. The microglial cells were divided into five groups; after the treatments, tests were performed to find a suitable BBR dose. (b) Investigating BBR-induced effects on proinflammatory factor releases. Cells were divided into four groups; after the treatments, IL-1β and IL-6 concentrations were measured. (c) Exploring the role of SOCS1 in the BBR-induced effects on microglial activation. Cells were divided into five groups; after the treatments, microglial M1/M2 activation markers were evaluated to assess microglial activation degree.
2.3. Western Blot Analysis
The N9 microglial cells were seeded into a 6-well cell culture plate at a density of 1 × 106 cells/well; after the treatments, the medium was removed and the cells were homogenized on ice with lysis buffer, containing 0.3 M sucrose, 0.15 M NaCl, 2 mM EDTA, 0.3 mM PMSF, 20 mM Tris-HCl, and 10 μg/ml leupeptin. Then, total protein was evaluated by using the Bradford method. And the Western blot analysis was performed as García-Bea et al. previously described [19]. The primary antibodies, including anti-iNOS (1 : 1000), anti-Arg-1 (1 : 1000), anti-SOCS1 (1 : 1000), anti-β-actin (1 : 1000), and anti-GAPDH (1 : 1000), were taken in this study. Chemiluminescence technique was used to assess the antigens of the cellular proteins. Computerized analysis software (Bio-Rad Laboratories, USA) was used to perform image analysis.
2.4. Enzyme-Linked Immunosorbent Assay
After the treatments, the supernatants of the cell culture plates were collected, and the concentrations of TNF-α, IL-1β, IL-6, BDNF, GDNF, and IL-10 were measured according to the manufacturers' instructions of the corresponding ELISA kits. The experiments were repeated three times, and the concentrations of the cytokines were expressed as picograms per litre.
2.5. Cell Viability Assay
The cells were plated into a 96-well plate at a density of 1 × 105 cells/well; after 24 h incubation, 20 μl MTT solution (5 mg/ml) was added into each well. And after 4 h incubation in the cell incubator at 37°C, the cell medium was removed; then, 150 μl DMSO was added into each well. After 15 min, formazan of each well was dissolved completely. The absorbance of each well was evaluated by using a spectrophotometer at a wavelength of 492 nm.
2.6. siRNA Interfering
To downregulate the microglial SOCS1 protein expression, the cells were exposed to 60 pmol SOCS1-siRNA or 60 pmol scrambled- (SC-) siRNA by using the Lipofectamine reagent (Invitrogen, USA) in serum-free medium, under the instructions of the manufacturers. And the cells were incubated for 6 h and then recovered for an extra 6 h before the treatments of the drugs. And the SC-siRNA treatment was considered the negative control.
2.7. Immunocytochemistry
N9 microglial cells were seeded into a confocal microscopy-specific cell culture dish at a density of 1 × 105 cells/well. After the treatments, the medium was removed, and the dish was washed three times with phosphate buffer saline (PBS) at room temperature (RT), five min/time. Then, the cells were fixed with 4% paraformaldehyde solution for 30 min; then, the cells were washed with PBS. Next, the cells were blocked with 5% bovine serum albumin (BSA) solution at 4°C after three times washing with PBS. After 1 h BSA blocking, the cells were incubated overnight at 4°C with a primary antibody (iNOS, 1 : 200; Arg-1, 1 : 200). Then, the cells were washed three times with PBS, 5 min/time. The cells were exposed to Cy3-labeled (red) or FITC-labeled (green) secondary antibody for 60 min at RT. At the end of the exposures, 150 μl DAPI solution was added into the dish; after 5 min incubation in the dark, the solution was removed, and the cells were washed three times with PBS, five min/time. Then, the cells were observed, and pictures were taken by using a confocal microscope (FV10i, Olympus, Japan).
2.8. Statistical Analysis
SPSS 20.0 for Windows was used to conduct the data of this study. And all data of this study were expressed as means ± Standard Deviation (SD). The results of each group were compared with One-way Analysis of Variance (ANOVA), followed by Tukey's multiple comparisons test. And P < 0.05 was considered to be significant.
3. Results
3.1. Berberine Downregulated iNOS Expression and TNF-α Release and Increased SOCS1 Level in Microglia Treated with Aβ
To explore a suitable dose of BBR, in this experiment, the N9 microglia were divided into the control group, Aβ exposure group (cells were treated with the medium containing 5 μM Aβ), 0.1 μM BBR+5 μM Aβ group (cells were treated with the medium containing 0.1 μM BBR plus 5 μM Aβ), 1 μM BBR+5 μM Aβ group, and 5 μM BBR+5 μM Aβ group; after incubation for 24 h, we took the Western blot analysis to assess iNOS and SOCS1 expression levels, and the TNF-α concentration in the medium was measured by using the ELISA (Figures 2(a) and 2(b)). Compared with the normal cultured cells of the control, Aβ exposure increased the iNOS expression and TNF-α release (P < 0.05) and did not cause marked change of the SOCS1 expression (Figure 2(c)); 1 μM and 5 μM BBR (but not 0.1 μM) significantly decreased the iNOS expression and TNF-α release and upregulated the SOCS1 expression (P < 0.05), indicating BBR can reverse the Aβ-induced microglial activation and increase SOCS1 expression. BBR of 5 μM was chosen in the next experiments.
Figure 2.
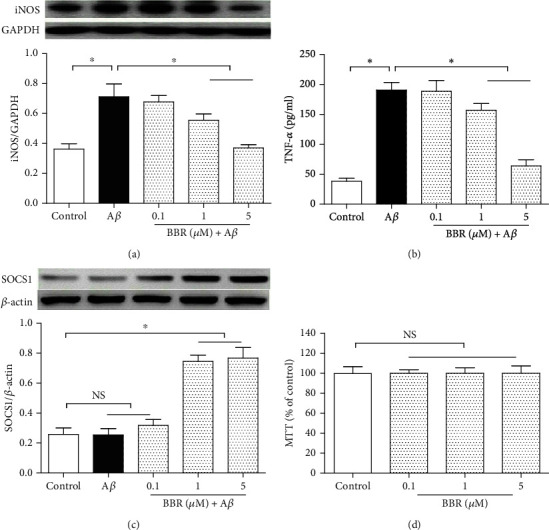
Berberine reduced iNOS expression and TNF-α release and upregulated SOCS1 expression in Aβ-treated microglial cells. The N9 microglia were divided into five groups, including the normal cultured control group, 5 μM Aβ group, and three concentrations of berberine treatment groups (medium containing 0.1 μM, 1 μM, or 5 μM berberine plus 5 μM Aβ); after 24 h treatment, iNOS and SOCS1 expressions were measured by using Western blot, and TNF-α release was assessed by ELISA. Then, the cells were divided into four groups, including the control and 0.1 μM, 1 μM, and 5 μM berberine treatment groups; after 24 h incubation, the MTT assay was performed to evaluate the cell injury level. (a) Microglial iNOS protein expression (n = 4). (b) TNF-α concentration in the medium (n = 8). (c) SOCS1 protein expression (n = 4). (d) Cell viability assay (n = 8). Results are expressed as means ± SD. ∗P < 0.05; NS: no significance.
In addition, to exclude the potential toxic effect caused by BBR in this study, microglia were divided into four groups (Figure 2(d)), including the normal cultured control and three BBR exposure groups; after a 24 h incubation, we took the MTT assay to check microglial viability. Compared with the control, the three doses of BBR did not induce obvious effects on cell viability (P > 0.05), showing that the BBR-induced anti-inflammations in this study were via pharmalogical effects, but not the toxic effects.
3.2. Berberine Inhibited Proinflammatory Factor Releases from Aβ-Treated Microglial Cells
To assess the proinflammatory factor releases in the presence of BBR, the grouping of microglia is shown in Figures 3(a) and 3(b). After treatment for 24 h, the two proinflammatory cytokines' levels, IL-1β and IL-6, were evaluated. Compared with microglia of the normal cultured control, Aβ of 5 μM obviously increased the two cytokines' releases (P < 0.05), and 5 μM BBR significantly reversed the Aβ-induced upregulations of the two proinflammatory factors' concentrations (P < 0.05), and administration alone with 5 μM BBR did not induce significant changes of the two proinflammatory factors' releases (P > 0.05).
Figure 3.
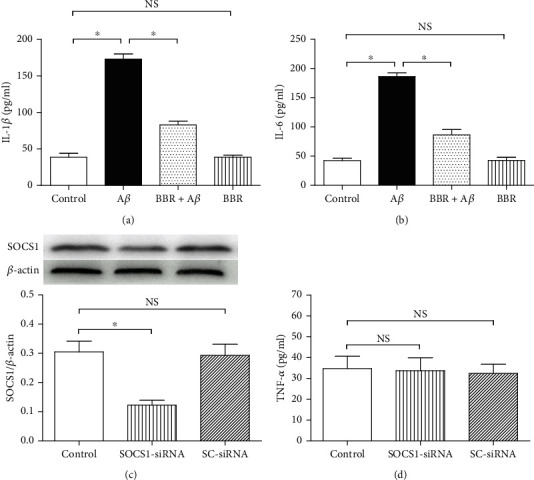
Berberine decreased IL-1β and IL-6 releases in Aβ-treated microglia, and SOCS1-siRNA or SC-siRNA did not induce obvious inflammation in untreated microglia. N9 microglial cells were divided into four groups, including the normal cultured control group, 5 μM Aβ exposure group, BBR treatment group (medium containing 5 μM BBR and 5 μM Aβ), and 5 μM BBR treatment group. After 24 h treatments, ELISA was taken to assess IL-1β and IL-6 concentrations in the medium. Then, the cells were divided into three groups, including control, SOCS1-siRNA, and SC-siRNA groups; after 6 h incubation, Western blot and ELISA were taken to evaluate the SOCS1 expression and TNF-α release. (a) IL-1β concentration in the medium (n = 8). (b) IL-6 concentration in the medium (n = 8). (c) SOCS1 expression level (n = 4). (d) The siRNA-induced effects on TNF-α release (n = 8). Results are expressed as means ± SD. ∗P < 0.05; NS: no significance.
To test the inhibitions of SOCS1 expression and cellular inflammation, we took specific siRNA to downregulate the SOCS1 protein expression, and the grouping of the cells was shown in the figures (Figures 3(c) and 3(d)), including the control, SOCS1 gene-specific small interfering RNA (SOCS1-siRNA), and scrambled small interfering RNA (SC-siRNA) groups; after exposure for 6 h, SOCS1 expression and TNF-α concentration in the supernatant were assessed. SOCS1-siRNA markedly downregulated the SOCS1 expression, compared with the cells of the control (P < 0.05); however, the SC-siRNA did not (P > 0.05); meanwhile, either the SOCS1-siRNA or the SC-siRNA did not bring about obvious change of the TNF-α concentration (P > 0.05). These observations above suggested that the SOCS1-siRNA used in this investigation was effective in downregulating SOCS1 expression without inducing additional inflammation in the cells.
3.3. SOCS1-siRNA Blocked BBR-Caused Inhibitions of Microglial iNOS Expression and Proinflammatory Factor Release
To investigate the role of SOCS1 in Aβ-treated microglial cells exposed to BBR, the SOCS1-siRNA was taken to downregulate SOCS1 protein expression. Compared with the control, 5 μM Aβ significantly increased iNOS expression (Figures 4(a) and 4(b)) and proinflammatory factor releases (P < 0.05), such as TNF-α, IL-1β, and IL-6 (Figures 4(c)–4(e)), and 5 μM BBR obviously inhibited the Aβ-induced upregulations of iNOS and the three inflammatory factors' releases above (P < 0.05); however, the SOCS1-siRNA markedly reversed the BBR-induced effects on the iNOS expression and the three cytokines' releases (P < 0.05); the SC-siRNA, however, did not (P > 0.05). These results above showed that BBR can decrease neuroinflammation, and microglial cell number of M1 activated status in Aβ-treated cells, and SOCS1 might mediate the effects.
Figure 4.
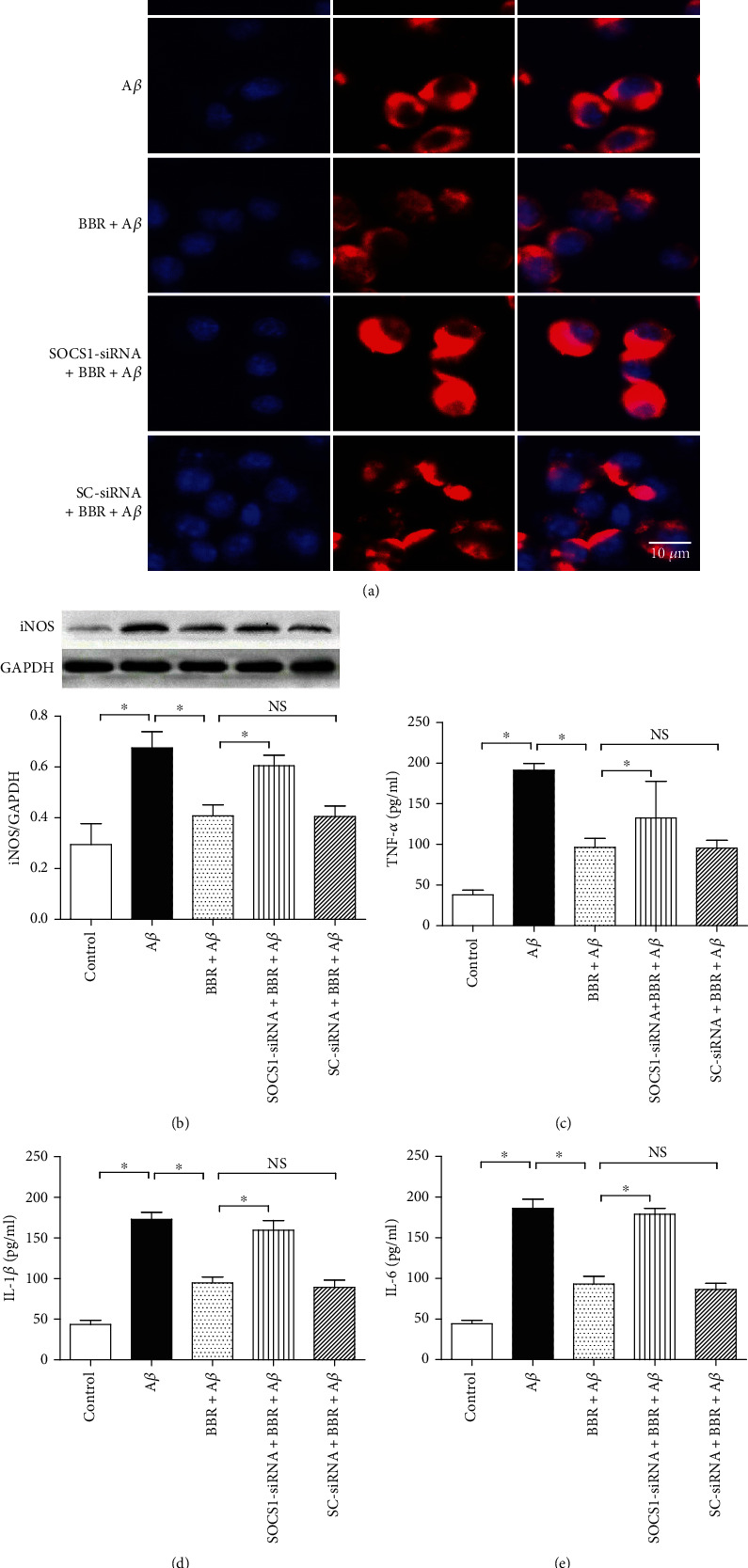
SOCS1-siRNA reversed BBR-induced effects on iNOS expression and proinflammatory factor releases. The cells were divided into five groups, including the normal cultured control group, 5 μM Aβ exposure group, BBR treatment group (medium containing 5 μM BBR and 5 μM Aβ), SOCS1 treatment group (microglial cells were treated with SOCS1-siRNA for 6 h and then exposed to the medium containing 5 μM BBR and 5 μM Aβ), and scrambled- (SC-) siRNA treatment group (microglial cells were treated with SC-siRNA for 6 h and then exposed to the medium containing 5 μM BBR and 5 μM Aβ); then, after 24 h treatment, iNOS expression was observed by using immunocytochemistry staining and Western blot, and inflammatory factors were assessed by using ELISA. (a) Immunocytochemistry staining of microglial iNOS expression. (b) Western blot result of iNOS expression (n = 4). (c–e) TNF-α, IL-1β, and IL-6 concentrations in the medium (n = 8). Results are expressed as means ± SD. Bar = 10 μm; ∗P < 0.05; NS: no significance.
3.4. SOCS1-siRNA Inhibited BBR-Caused Upregulations of Microglial Arg-1 Level and Anti-Inflammatory Cytokine Secretion
To explore the BBR-induced modulations in microglial M2 activated state and also investigate SOCS1 in the process, bioactive markers of M2 microglial cells, including Arg-1 level and BDNF, GDNF, and IL-10 releases, were measured. In Figure 5, compared with the control group, 5 μM Aβ did not cause marked changes in Arg-1 expression (Figures 5(a) and 5(b)) and BDNF and GDNF concentrations (Figures 5(c) and 5(d)), and 5 μM BBR markedly increased Arg-1 expression and the above two neurotrophic factor, BDNF and GDNF, secretions (P < 0.05); however, SOCS1-siRNA (P <0.05), but not the SC-siRNA (P > 0.05), obviously blocked the BBR-induced upregulations of Arg-1 expression and BDNF and GDNF releases. Interestingly, the concentrations of IL-10 did not change significantly (P > 0.05) in the supernatant, and elevated IL-10 concentration can induce anti-inflammatory effect. These results showed that BBR can increase neurotrophic factor releases and the microglial cell number of M2 activated status in Aβ-treated cells, and SOCS1 might mediate the anti-inflammation of BBR.
Figure 5.

SOCS1-siRNA reversed BBR-induced effects on Arg-1 expression and neurotrophic factor releases. The cells were divided into five groups, including the normal cultured control group, 5 μM Aβ treatment group, BBR treatment group (medium containing 5 μM BBR and 5 μM Aβ), SOCS1 treatment group (microglial cells were treated with SOCS1-siRNA for 6 h and then exposed to the medium containing 5 μM BBR and 5 μM Aβ), and scrambled- (SC-) siRNA treatment group (microglial cells were treated with SC-siRNA for 6 h and then exposed to the medium containing 5 μM BBR and 5 μM Aβ); then, after the treatments, Arg-1 expression was measured by using immunocytochemistry staining and Western blot, and neurotrophic factors were assessed by using ELISA. (a) Immunocytochemistry staining of microglial Arg-1 expression. (b) Western blot result of Arg-1 expression (n = 4). (c–e) BDNF, GDNF, and IL-10 concentrations in the medium (n = 8). Results are expressed as means ± SD. Bar = 10 μm; ∗P < 0.05; NS: no significance.
3.5. Berberine Upregulated the SOCS1 Level in N9 Microglia, Which Was Reversed by SOCS1-siRNA
In Figure 6, compared with the control, 5 μM Aβ treatment for 24 h did not upregulate SOCS1 protein expression (P > 0.05), and a dose of 5 μM BBR markedly increased the protein expression of SOCS1; the SOCS1-siRNA, however, obviously inhibited the BBR-induced upregulation of SOCS1 (P < 0.05), and SC-siRNA did not cause marked changes of the BBR-induced SOCS1 upregulation (P > 0.05).
Figure 6.
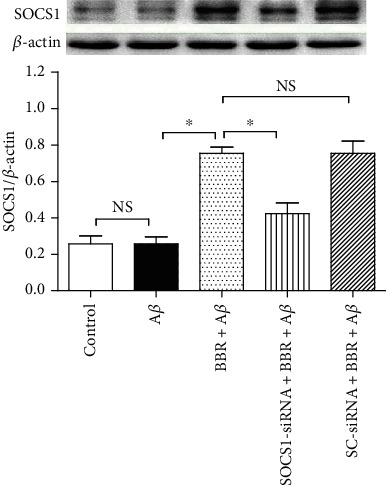
SOCS1-siRNA reversed the BBR-induced effect on SOCS1 protein expression. The cells were divided into five groups, and the treatments were the same as Figure 5, and Western blot was taken to assess SOCS1 protein expression (n = 4). Results are expressed as means ± SD. ∗P < 0.05; NS: no significance.
4. Discussion
In this investigation, we observed that treatment with 5 μM Aβ for 24 h increased microglial markers of M1 activated state in N9 microglial cells, including iNOS expression and proinflammatory factors' releases, and the presence of 5 μM BBR reduced the markers' levels of the microglial M1 activated status above, meanwhile upregulating the markers' levels of microglial M2 activated status and the SOCS1 expression, such as Arg-1 expression and two neurotrophic factors' concentrations (BDNF and GDNF) in the medium. Yet, silencing SOCS1 protein expression by using specific siRNA, but not the scrambled siRNA, significantly blocked the BBR-caused changes of biomarkers of microglial M1/M2 status and the SOCS1 expression. These observations showed that BBR inhibits microglial activation caused by Aβ via shifting microglia from M1 to M2 status, and SOCS1 protein might modulate the anti-inflammation of BBR.
AD is an extremely common disorder of the central nervous system, which is age-related and brings about progressive neurological dysfunctions. And it is estimated that about 5 million patients with an age greater than or equal to 65 years are suffering from AD, and by 2050, the total number of AD cases will be at least 13.8 million [20]. Currently, the US Food and Drug Administration (FDA) approved just two medicines, acetylcholinesterase inhibitors and N-methyl-D-aspartate receptor antagonists. However, because of serious side effects and limitations, the two kinds of drugs are rarely prescribed [21, 22]. For this reason, searching for effective medicine or therapy for AD is of great emergency. Cerebral Aβ accumulation is a main biological character of AD, and overdose of Aβ induces neural impairment through two ways. Firstly, overdose of Aβ can injure neuronal cells directly and induces neuron death and neurological disabilities; secondly, overdose of Aβ can stimulate and activate glial cells, including astrocytes and microglial cells; the activated microglia or astrocytes can produce proinflammatory factors and lead to neuroinflammation, and chronic or acute neuroinflammation can also bring about neuronal injury and neurological dysfunctions finally [23, 24]. Therefore, reducing Aβ-induced neuroinflammation is a key step for controlling AD progression. In the present investigation, N9 microglial cells were treated with Aβ to bring about microglial activation, and this cell injury model was used widely in investigating the neuroinflammation of AD [25]. In fact, according to the latest findings of many studies about microglial cells, which could be activated into two different statuses, classic activated state (M1 state) or alternative activated state (M2 state), microglial of M1 status can release a variety of proinflammatory factors and enhance the neuroinflammation; therefore, microglial cells of this state can injure the brain tissue; on the other hand, microglial cells of M2 status could generate a lot of neurotrophic and anti-inflammatory cytokines and reduce the neuroinflammation and increase neural regeneration, so microglial cells of this status are considered to be beneficial [10–12]. For the reasons mentioned above, modulating M1/M2 state or enhancing the microglial M1/M2 state shift is a key method in inhibiting microglial activation-induced neurological disorders.
In China and some other Asian countries, BBR is used universally as a herb for hepatological disorders, microbial infection, and skin infection for many decades [26, 27]. Recently, many investigations indicated that BBR is effective in treating some neurodegenerative diseases. In a scopolamine-induced memory loss model, BBR reduces proinflammatory cytokine release and increases neurotrophic factor generations, including BDNF, GDNF, and cAMP-response element-binding protein (CREB) [28]. In a cell injury model of Aβ-induced BV-2 microglial activation, BBR pretreatment decreased cyclooxygenase-2 (COX-2) and iNOS protein expression levels and also inhibited the productions of monocyte chemotactic protein-1 and IL-6, which are hi-related to inflammatory response [29]. This evidence indicates that BBR can inhibit AD progression by controlling Aβ-induced neuroinflammation. In this investigation, we observed that BBR reduced the markers' levels of microglial M1 state in Aβ-treated N9 microglial cells and also increased the microglial M2 markers' levels in the cells, including BDNF, GDNF, and Arg-1 protein expression, suggesting that BBR can ameliorate the M1/M2 ratio in Aβ-stimulated microglial cells. The findings of our study are similar to the findings mentioned above. However, the exact anti-inflammatory mechanism of BBR in neuronal cells is still not clear. SOCS is not just a protein molecule, which consists of 7 SOCS molecules, from SOCS1 to SOCS7, and the cytokine-inducible SH2 protein (CIS). Being different from the other SOCS molecules, both SOCS1 and SOCS3 could be found in microglia. In addition, many investigations indicated that upregulation of SOCS1 protein can modulate microglial M1/M2 activated state; therefore, we explored the role of SOCS1 rather than the other members of SOCS proteins. And we observed that BBR treatment upregulated SOCS1 expression and inhibited the changes of microglial M1 and M2 states' markers induced by Aβ, yet SOCS1-siRNA obviously blocked the BBR-caused changes of the SOCS1 expression and the microglial M1/M2 state markers, but SOCS1-siRNA or SC-siRNA alone did not induce obvious inflammation in N9 microglial cells, indicating that the BBR-induced anti-inflammation is via inhibiting SOCS1, but not the siRNA molecule. Interestingly, the concentration of IL-10 in the supernatant did not vary significantly in the presence of BBR, indicating that the BBR-induced anti-inflammation is via reducing inflammatory cytokine secretion and elevating neurotrophic factors' releases, but not by enhancing the anti-inflammatory cytokine secretion. And in some other studies, as a main ingredient of San-Huang-Xie-Xin-Tang, a formula of Traditional Chinese Medicine, BBR is used in treating cardiovascular and neurodegenerative disorders. BBR can also improve memory and spatial learning ability by increasing Aβ metabolism [30]. Because microglia are the main cells breaking Aβ down in the brain, enhancing Aβ clearance might be another neuroprotective mechanism of BBR in AD. These observations of this study explained, to some extent, the BBR-induced anti-inflammatory mechanism in AD and offered a novel therapeutic target in treating AD. But there are still some limitations to the study. In the first place, the results of this investigation are completely from in vitro experiments, so in vivo experiments and clinical studies are absolutely necessary to test and verify the results of this study. Secondly, we just investigated SOCS1 in the BBR-caused anti-inflammatory effects on Aβ-treated microglia; whether other SOCS proteins are involved in the BBR-caused anti-inflammation is still not clear.
5. Conclusions
In summary, in this investigation, we found that BBR decreases microglial activation by improving M1/M2 status in Aβ-treated N9 microglial cells, and SOCS1 protein may mediate the effects.
Acknowledgments
This research was funded by the Natural Science Basic Research Program of Shaanxi Province (Program No. 2020JQ-559).
Data Availability
The data of this investigation were kept by the corresponding author (He Bao; baohe2yuan@xjtu.edu.cn). If some researchers are in need, they can achieve the data by sending an e-mail to the corresponding author.
Conflicts of Interest
The authors of this study declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contributions
Qi Guo and Chen Wang contributed equally to this work.
References
- 1.Solis E., Hascup K. N., Hascup E. R. Alzheimer’s disease: the link between amyloid-β and neurovascular dysfunction. Journal of Alzheimer's Disease . 2020;76(4):1179–1198. doi: 10.3233/JAD-200473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian J., Betensky R. A., Hyman B. T., Serrano-Pozo A. Association ofAPOEgenotype with heterogeneity of cognitive decline rate in Alzheimer disease. Neurology . 2021;96(19):e2414–e2428. doi: 10.1212/WNL.0000000000011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalamägi J., Lavikainen P., Taipale H., et al. Predictors of high hospital care and medication costs and cost trajectories in community-dwellers with Alzheimer's disease. Annals of Medicine . 2019;51(5-6):294–305. doi: 10.1080/07853890.2019.1642507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leuzy A., Ashton N. J., Mattsson-Carlgren N., et al. 2020 update on the clinical validity of cerebrospinal fluid amyloid, tau, and phospho-tau as biomarkers for Alzheimer's disease in the context of a structured 5-phase development framework. European Journal of Nuclear Medicine and Molecular Imaging . 2021;48(7):2121–2139. doi: 10.1007/s00259-021-05258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira J. B., Janelidze S., Ossenkoppele R., et al. Untangling the association of amyloid-β and tau with synaptic and axonal loss in Alzheimer's disease. Brain . 2021;144(1):310–324. doi: 10.1093/brain/awaa395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Y., Wu X., Li X., et al. TREM2 is a receptor for β-amyloid that mediates microglial function. Neuron . 2018;97(5):1023–1031.e7. doi: 10.1016/j.neuron.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Frailes C., di Lauro C., Bianchi C., et al. Amyloid peptide induced neuroinflammation increases the P2X7 receptor expression in microglial cells, impacting on its functionality. Frontiers in Cellular Neuroscience . 2019;13, article 143 doi: 10.3389/fncel.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song S., Jiang L., Oyarzabal E. A., et al. Loss of brain norepinephrine elicits neuroinflammation-mediated oxidative injury and selective caudo-rostral neurodegeneration. Molecular Neurobiology . 2019;56(4):2653–2669. doi: 10.1007/s12035-018-1235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvo-Rodríguez M., de la Fuente C., García-Durillo M., García-Rodríguez C., Villalobos C., Núñez L. Aging and amyloid β oligomers enhance TLR4 expression, LPS-induced Ca2+ responses, and neuron cell death in cultured rat hippocampal neurons. Journal of Neuroinflammation . 2017;14(1, article 24) doi: 10.1186/s12974-017-0802-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia J., Peng J., Li Z., et al. Cannabinoid CB2 receptor mediates nicotine-induced anti-inflammation in N9 microglial cells exposed to β amyloid via protein kinase C. Mediators of Inflammation . 2016;2016:10. doi: 10.1155/2016/4854378.4854378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shan M., Lin S., Li S., et al. TIR-domain-containing adapter-inducing interferon-β (TRIF) is essential for MPTP-induced dopaminergic neuroprotection via microglial cell M1/M2 modulation. Frontiers in Cellular Neuroscience . 2017;11, article 35 doi: 10.3389/fncel.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin X., Zhan J., Jiang J., Ren Y. Upregulation of neuronal cylindromatosis expression is essential for electroacupuncture-mediated alleviation of neuroinflammatory injury by regulating microglial polarization in rats subjected to focal cerebral ischemia/reperfusion. Journal of Inflammation Research . 2021;Volume 14:2061–2078. doi: 10.2147/JIR.S307841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carniglia L., Ramírez D., Durand D., et al. Neuropeptides and microglial activation in inflammation, pain, and neurodegenerative diseases. Mediators of Inflammation . 2017;2017:23. doi: 10.1155/2017/5048616.5048616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S., Gao L., Liu X., Lu T., Xie C., Jia J. Resveratrol attenuates microglial activation via SIRT1-SOCS1 pathway. Evidence-Based Complementary and Alternative Medicine . 2017;2017:10. doi: 10.1155/2017/8791832.8791832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernández C., Bogdanov P., Gómez-Guerrero C., et al. SOCS1-derived peptide administered by eye drops prevents retinal neuroinflammation and vascular leakage in experimental diabetes. International Journal of Molecular Sciences . 2019;20(15, article 3615) doi: 10.3390/ijms20153615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., Tu S., Yang S., et al. Berberine modulates LPA function to inhibit the proliferation and inflammation of FLS-RA via p38/ERK MAPK pathway mediated by LPA1. Evidence-Based Complementary and Alternative Medicine . 2019;2019:12. doi: 10.1155/2019/2580207.2580207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao C., Su P., Lv C., et al. Berberine alleviates amyloid β-induced mitochondrial dysfunction and synaptic loss. Oxidative Medicine and Cellular Longevity . 2019;2019:11. doi: 10.1155/2019/7593608.7593608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan N. N., Cai C. Z., Wu M. Y., Su H. X., Li M., Lu J. H. Neuroprotective effects of berberine in animal models of Alzheimer's disease: a systematic review of pre-clinical studies. BMC Complementary and Alternative Medicine . 2019;19(1, article 109) doi: 10.1186/s12906-019-2510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Bea A., Walker M. A., Hyde T. M., Kleinman J. E., Harrison P. J., Lane T. A. Metabotropic glutamate receptor 3 (mGlu3; mGluR3; GRM3) in schizophrenia: antibody characterisation and a semi-quantitative western blot study. Schizophrenia Research . 2016;177(1-3):18–27. doi: 10.1016/j.schres.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shal B., Ding W., Ali H., Kim Y. S., Khan S. Anti-neuroinflammatory potential of natural products in attenuation of Alzheimer's disease. Frontiers in Pharmacology . 2018;9(548) doi: 10.3389/fphar.2018.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auld D. S., Kornecook T. J., Bastianetto S., Quirion R. Alzheimer's disease and the basal forebrain cholinergic system: relations to β-amyloid peptides, cognition, and treatment strategies. Progress in Neurobiology . 2002;68(3):209–245. doi: 10.1016/S0301-0082(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 22.Kumar A., Singh A. A review on Alzheimer's disease pathophysiology and its management: an update. Pharmacological Reports . 2015;67(2):195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Ali F., Baringer S. L., Neal A., Choi E. Y., Kwan A. C. Parvalbumin-positive neuron loss and amyloid-β deposits in the frontal cortex of Alzheimer's disease-related mice. Journal of Alzheimer's Disease . 2019;72(4):1323–1339. doi: 10.3233/JAD-181190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cisternas P., Taylor X., Lasagna-Reeves C. A. The amyloid-tau-neuroinflammation axis in the context of cerebral amyloid angiopathy. International Journal of Molecular Sciences . 2019;20(24, article 6319) doi: 10.3390/ijms20246319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai H., Liang Q., Ge G. Gypenoside attenuates β amyloid-induced inflammation in N9 microglial cells via SOCS1 signaling. Neural Plasticity . 2016;2016:10. doi: 10.1155/2016/6362707.6362707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Z., He B., Chen Z., Yan M., Wu L. Anti-inflammatory activity of berberine in non-alcoholic fatty liver disease via the Angptl2 pathway. BMC Immunology . 2020;21(1, article 28) doi: 10.1186/s12865-020-00358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X., Dai K., Liu C., et al. Berberine-coated biomimetic composite microspheres for simultaneously hemostatic and antibacterial performance. Polymers . 2021;13(3, article 360) doi: 10.3390/polym13030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S. Y., Gao Y., Sun J. Y., et al. Traditional Chinese medicine: role in reducing β-amyloid, apoptosis, autophagy, neuroinflammation, oxidative stress, and mitochondrial dysfunction of Alzheimer's disease. Frontiers in Pharmacology . 2020;11, article 497 doi: 10.3389/fphar.2020.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C. C., Hung T. H., Lee C. Y., et al. Berberine protects against neuronal damage via suppression of glia-mediated inflammation in traumatic brain injury. PLoS One . 2014;9(12, article e115694) doi: 10.1371/journal.pone.0115694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedemann T., Schumacher U., Tao Y., Leung A. K. M., Schröder S. Neuroprotective activity of coptisine from Coptis chinensis (Franch) Evidence-Based Complementary and Alternative Medicine . 2015;2015:9. doi: 10.1155/2015/827308.827308 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this investigation were kept by the corresponding author (He Bao; baohe2yuan@xjtu.edu.cn). If some researchers are in need, they can achieve the data by sending an e-mail to the corresponding author.


