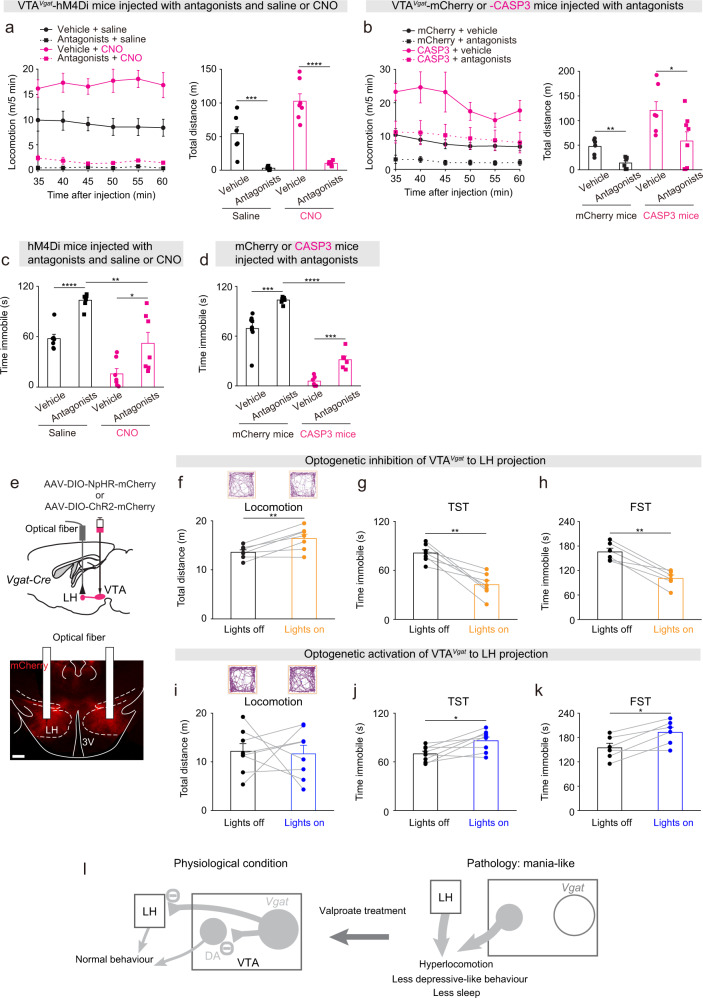
Fig. 5. Circuit mechanisms underlying the mania-like behaviors mediated by VTAVgat neurons.
a Locomotion speed and distance traveled of VTAVgat-hM4Di mice that received injections with vehicle or a dopamine receptor antagonist mixture (SCH-23390 and raclopride for D1 and D2/D3 receptors, respectively) and either saline (n = 7 mice) or CNO (n = 7 mice). Two-way ANOVA and Bonferroni-Holm post hoc test. F(saline or CNO) = 13.6; F(vehicle or antagonists) = 91.2; F(interaction) = 7.4; Vehicle + saline vs. antagonists + saline t(12) = 5, ***p = 0.0002; Vehicle + CNO vs. antagonists + CNO t(12) = 8.34, ****p = 2E−6. All error bars represent the SEM. b Locomotion speed and distance traveled of VTAVgat-mCherry and VTAVgat-CASP3 mice that received injections with either vehicle (n = 6 mice for mCherry and n = 10 mice for CASP3) or a dopamine receptor antagonist mixture (n = 6 mice for mCherry and n = 13 mice for CASP3). Two-way ANOVA and Bonferroni-Holm post hoc test. F(mCherry or CASP3) = 10.8; F(saline or antagonists) = 4.5; F(interaction) = 0.03; mCherry + vehicle vs. mCherry + antagonists t(10) = 4.14, **p = 0.002; CASP3 + vehicle vs. CASP3 + antagonists t(21) = 1.64, p = 0.11. All error bars represent the SEM. c Time spent immobile for the tail suspension assay of VTAVgat-hM4Di mice that received injections of either vehicle or the dopamine receptor antagonist mixture and saline or CNO. Two-way ANOVA and Bonferroni–Holm post hoc test. F(saline or CNO) = 36; F(Vehicle or antagonists) = 27; F(interaction) = 0.35; vehicle + saline vs. antagonists + saline t(12) = −7.59, ****p = 6E−6; vehicle + CNO vs. antagonists + CNO t(12) = −2.54, *p = 0.02; antagonists + saline vs. antagonists + CNO t(12) = 3.8, **p = 0.002. All error bars represent the SEM. d Time spent immobile for the tail suspension assay of VTAVgat-mCherry and VTAVgat-CASP3 mice that received injections of either vehicle (n = 8 mice for mCherry and n = 9 mice for CASP3) or the dopamine receptor antagonist mixture (n = 8 mice for mCherry and n = 9 mice for CASP3). Two-way ANOVA and Bonferroni-Holm post hoc test. F(mCherry or CASP3) = 209; F(saline or antagonists) = 25; F(interaction) = 4.38; mCherry + vehicle vs. mCherry + antagonists t(14) = −4.87, ***p = 0.0002; CASP3 + vehicle vs. CASP3 + antagonists t(16)=−2.16, *p = 0.04; mCherry + antagonists vs. CASP3 + antagonists t(15) = 13, ****p = 7e−10. All error bars represent the SEM. e AAV-DIO-NpHR-mCherry was injected the VTA of Vgat-ires-cre mice, and optic fibers were bilaterally implanted above the LH. The NpHR-mCherry fibers projecting from the VTAVgat neurons into the LH were stained by immunohistochemistry (mCherry, red). Scale bar: 200 μm. f Video-tracked paths and distance traveled in the open field by VTAVgat-NpHR mice (n = 7 mice) with and without opto-inhibition of VTAVgat terminals in the LH over 5 minute period. Paired t-test, t(6) = −4.08, **p = 0.006. g Time spent immobile on the tail suspension test (TST) of VTAVgat-NpHR mice (n = 7 mice) with and without opto-inhibition of VTAVgat terminals in the LH. Paired t-test, t(6) = 5.3, **p = 0.001. h Time spent immobile on the forced swimming test (FST) of VTAVgat-NpHR mice (n = 6 mice) with and without opto-inhibition of VTAVgat terminals in the LH. Paired t-test, t(5) = 6.77, **p = 0.001. i Video-tracked paths and distance traveled in the open field by VTAVgat-ChR2 mice (n = 8 mice) with and without opto-activation of VTAVgat terminals in the LH over 5-minute period. Paired t-test, t(7) = 0.18, p = 0.85. j Time spent immobile on the tail suspension test (TST) of VTAVgat-ChR2 mice (n = 8 mice) with and without opto-activation of VTAVgat terminals in the LH. Paired t-test, t(7) = −3.11, *p = 0.01. k Time spent immobile on the forced swimming test (FST) of VTAVgat- ChR2 mice (n = 6 mice) with and without opto-activation of VTAVgat terminals in the LH. Paired t-test, t(5) = −4, *p = 0.01. l Conceptual circuit diagram illustrating VTAVgat neurons inhibiting VTA dopamine (DA) neurons and circuitry in the LH. When the VTAVgat cells have acutely diminished or absent function, activity of VTA dopamine neurons and arousal-promoting neurons in the LH increase. Valproate can reverse the effects of these changes.