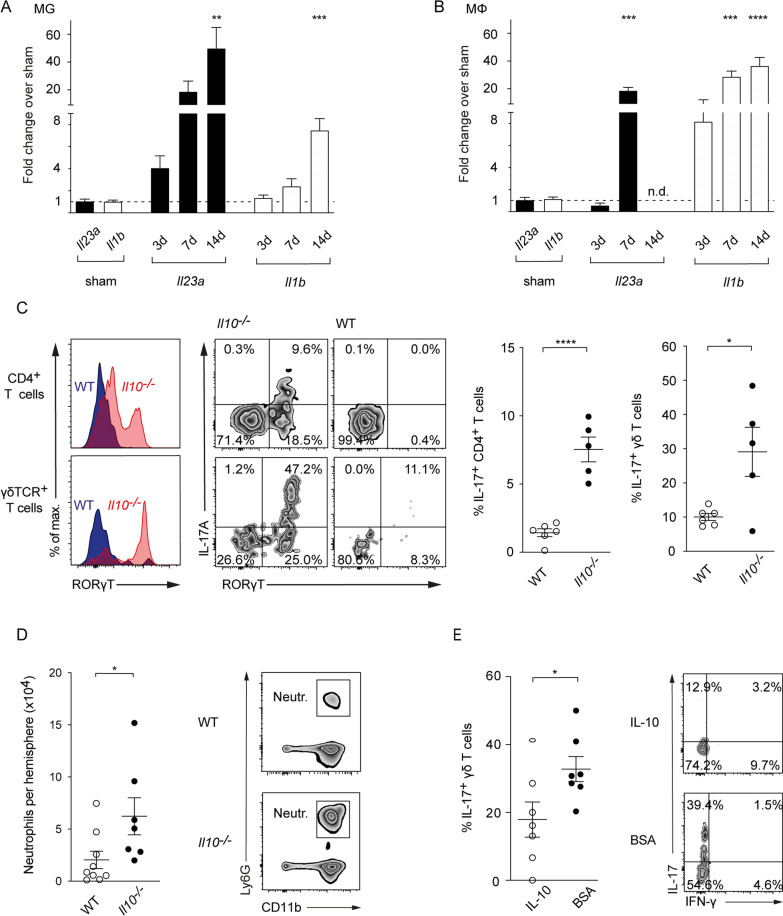
Fig. 3.
IL-10 controls the IL-17A axis in the proinflammatory milieu of the postischemic brain. Relative gene expression of Il23a and Il1b in brain resident CD45intermed/CD11b+ microglia (A) and central nervous system-infiltrating CD45high/CD11b+/CD11c−/MHCII−/Ly6g−/F4/80+ macrophages (B) purified 3, 7 and 14 days after tMCAO by fluorescence activated cell sorting from ischemic hemispheres. Expression levels were normalized to corresponding levels of splenic macrophages and microglia after sham operation. C Flow cytometric analysis of IL-17A produced by CD4+ and γδ T cells isolated from ischemic hemispheres of WT controls and Il10−/− mice 7 days after tMCAO. D Flow cytometric analysis of number of infiltrating neutrophils in the ischemic hemispheres of WT controls and Il10−/− mice 3 days after tMCAO. E Frequency of IL-17A producing γδ T cells purified from ischemic brains was analyzed 3 days following tMCAO in control mice (BSA) and mice receiving IL-10 intracerebral 3 h after tMCAO induction. A, B RT-qPCR gene expression data is represented as mean ± SEM of 4–7 WT, Flow cytometric data as mean ± SEM of 6 WT and 5 Il10−/− (C), 9 WT and 7 Il10−/− mice (D), and of 7 WT mice in each treatment group (E). Statistical significances were analyzed by one-way ANOVA with Bonferroni post hoc test (A, B) and by Student t test (C–E). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001