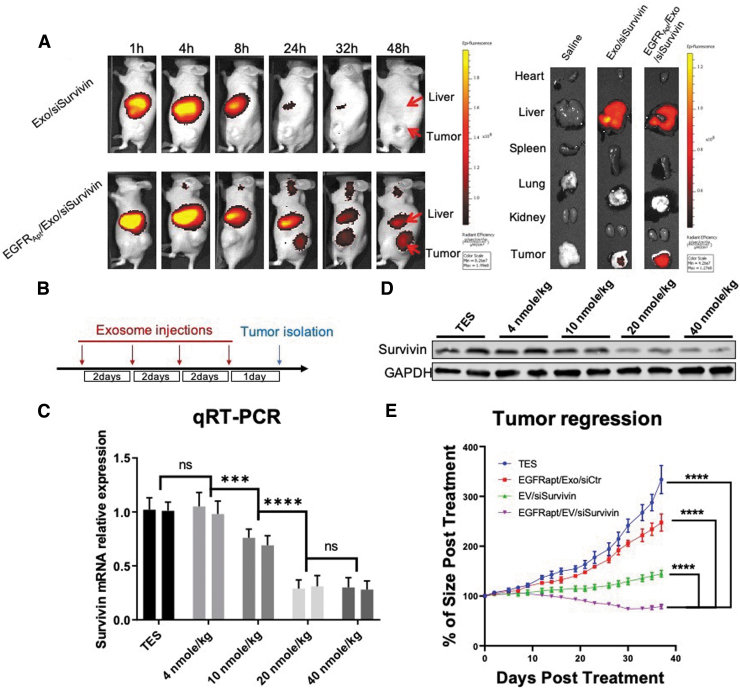
FIG. 6.
In vivo evaluation of EGFRApt/Exo/siSurvivin on NSCLC tumor xerograph mice. (A) Biodistribution study by tracing Alexa750-labeled siSurvivin delivered by exosomes. H596 NSCLC cell-developed tumor xenograft mice were I.V. administrated with 100 μL of 20 μM of RNA Alexa750-labeled siSurvivin loaded in exosomes or EGFRApt/Exo and imaging by IVIS post 1, 4, 8, 24, 32, and 48 h shown on the left. Organ was dissected at 48 h and imaged by IVIS shown on the right. (B) Scheme of exosome injection and tumor isolation time frame for dose optimization study. (C) mRNA level of survivin gene in tumor quantified by qRT-PCR assay post three repeated I.V. administration of EGFRApt/Exo/siSurvivin in 4, 10, 20, and 40 nmol/kg of siRNA concentration and TES buffer on H596 NSCLC cell-developed tumor xenograft mice, n = 3, error bar ± SD. Protein level assayed by western blot shown in (D). (E) Tumor growth curve traced during six repeated I.V. administration of EGFRApt/Exo/siSurvivin, EGFRApt/Exo/siCtr, Exo/siSurvivin, and TES buffer in 40 nmol/kg of siRNA concentration on H596 NSCLC cell-developed tumor xenograft mice. ***P < 0.001, ****P < 0.0001, two-way ANOVA (mix model repeated measurement), n = 5, error bar ± SEM. ANOVA, analysis of variance; I.V., intravenus; IVIS, in vivo imaging system; ns, no significance.